The recent identification of agents that have significantly influenced the therapy of clear cell renal carcinoma and the decreasing size of renal masses, usually detected serendipitously, have led to a resurgence in imaging for this condition. Although structural methods continue to be used routinely for identification of renal masses, functional and molecular techniques are showing considerable promise in their ability to characterize unique features of the renal cancer phenotype. This article discusses the evolving role of molecular imaging in the evaluation of renal cancer, including current and future applications.
The recent identification of agents that have significantly influenced the therapy of clear cell renal carcinoma and the decreasing size of renal masses, usually detected serendipitously, have led to a resurgence in imaging for this condition. Although structural methods continue to be used routinely for identification of renal masses, functional and molecular techniques are showing considerable promise in their ability to characterize unique features of the renal cancer phenotype. This article discusses the evolving role of molecular imaging in the evaluation of renal cancer, including current and future applications.
Imaging of renal masses
The average size of detected renal masses, which now usually are detected serendipitously, has decreased considerably in the past decade. This decrease in size has increased the need for characterization of the masses detected, because management is heavily influenced by the nature of the lesion. Because most lesions are detected and then evaluated using structural imaging methodologies, particularly ultrasound, CT, and MRI, attempts have been made to use these modalities to characterize renal masses. All these techniques seem to be useful, especially in centers with experience. All these modalities, however, including newer techniques such as dynamic contrast-enhanced MRI and measurement of tumor vascularity, are in essence structural or at best functional imaging techniques that seek to characterize renal masses based on radiodensity or blood flow.
Positron emission tomography
Positron emission tomography (PET) has improved tremendously the ability to characterize the cancer phenotype. [18F]-fluorodeoxyglucose (FDG) is an excellent surrogate for glucose use, which is increased in most cancers. Renal excretion of FDG limits its usefulness in the detection of primary tumors. It is, however, an excellent agent for staging and for detection of metastases. Tumor cells constitutively use glucose (the Warburg effect) and hence demonstrate greater FDG uptake than normal cells, appearing as areas of increased FDG accumulation.
PET is a nuclear medicine technique that relies on the detection of energy emitted from radiotracers exogenously administered to patients. Tracers are substances that, in very low concentrations, are able to evaluate biologic functions without disturbing the milieu, a principle pioneered by Hevesy in 1935.
PET images result from the detection of co-incident 511-keV gamma rays originating from the annihilation of the emitted positron and a neighboring electron. These gamma rays are detected by an array of scintillation crystals, localizing the event in a linear trajectory within the patient.
PET is the most sensitive imaging tool used routinely in clinical practice, with sensitivity in the order of picomolar concentrations. Tracer uptake is indicated by the standardized uptake value, a measure of mean tissue tracer concentration relative to the mean whole body tracer concentration.
PET has revolutionized the ability to study tumor biology. PET, using different radiotracers, can evaluate cellular metabolism, hypoxia, and the expression of cell surface markers. PET cannot distinguish between various positron-emitting radioisotopes, and therefore only one PET tracer can be studied at a given time. Most positron-emitting radioisotopes have short half-lives, however, and thus serial studies are feasible.
Because PET essentially identifies molecular features of cellular processes, it can be thought of as the pre-eminent molecular imaging modality. An overview of PET imaging of renal cancer follows.
Positron emission tomography
Positron emission tomography (PET) has improved tremendously the ability to characterize the cancer phenotype. [18F]-fluorodeoxyglucose (FDG) is an excellent surrogate for glucose use, which is increased in most cancers. Renal excretion of FDG limits its usefulness in the detection of primary tumors. It is, however, an excellent agent for staging and for detection of metastases. Tumor cells constitutively use glucose (the Warburg effect) and hence demonstrate greater FDG uptake than normal cells, appearing as areas of increased FDG accumulation.
PET is a nuclear medicine technique that relies on the detection of energy emitted from radiotracers exogenously administered to patients. Tracers are substances that, in very low concentrations, are able to evaluate biologic functions without disturbing the milieu, a principle pioneered by Hevesy in 1935.
PET images result from the detection of co-incident 511-keV gamma rays originating from the annihilation of the emitted positron and a neighboring electron. These gamma rays are detected by an array of scintillation crystals, localizing the event in a linear trajectory within the patient.
PET is the most sensitive imaging tool used routinely in clinical practice, with sensitivity in the order of picomolar concentrations. Tracer uptake is indicated by the standardized uptake value, a measure of mean tissue tracer concentration relative to the mean whole body tracer concentration.
PET has revolutionized the ability to study tumor biology. PET, using different radiotracers, can evaluate cellular metabolism, hypoxia, and the expression of cell surface markers. PET cannot distinguish between various positron-emitting radioisotopes, and therefore only one PET tracer can be studied at a given time. Most positron-emitting radioisotopes have short half-lives, however, and thus serial studies are feasible.
Because PET essentially identifies molecular features of cellular processes, it can be thought of as the pre-eminent molecular imaging modality. An overview of PET imaging of renal cancer follows.
Glucose metabolism
FDG is the most frequently used PET radiotracer and is the only one approved by the Food and Drug Administration (FDA) for oncologic PET imaging. Like glucose, FDG is transported into cells by glucose transporters and is phosphorylated by hexokinase. It is not metabolized further, however, and thus it accumulates inside cells. Tumor cells use glucose constitutively, as described by Warburg, and hence demonstrate greater FDG uptake, appearing as areas of increased FDG accumulation.
FDG-PET is being used increasingly in staging and response assessment in a variety of cancers, such as lung, breast, lymphoma, melanoma, and neoplasms of the gastrointestinal tract. Its role in renal cancer is still controversial, however.
FDG-PET reportedly has limited use in the primary detection and staging of renal cell carcinoma. Because FDG is excreted primarily through the urine, foci of increased uptake may be mistaken as activity in the collecting system. Hydration and diuretics potentially can be used to avoid such confounding factors, as is done in bladder cancer. In addition, hybrid imaging with PET/CT scanners also has the potential to improve the sensitivity of FDG-PET in renal tumors from the levels initially reported.
Bachor and colleagues initially described FDG-PET as having an overall sensitivity of 77% in detecting the primary tumor. From a group of 26 patients who had pathologically proven renal cell carcinomas, 20 were identified by FDG-PET; the diagnostic accuracy depended on the degree of tumor differentiation. Most importantly, FDG-PET proved to be a very accurate method for lymph node staging, with no false negatives in this study.
Goldberg and colleagues used FDG-PET to study patients who had renal tumors and indeterminate renal cysts. The authors found that in 9 of 10 patients FDG-PET depicted solid neoplasms accurately. Except for one patient who had a renal cyst and a 4-mm papillary neoplasm, all benign lesions also were classified as such by FDG-PET.
Aide and colleagues prospectively evaluated the ability of FDG-PET to characterize renal cancers and to detect distant metastasis in treatment-naïve and postnephrectomy patients. FDG-PET was less accurate than CT in imaging the primary tumors but had a greater sensitivity in detecting metastatic disease.
In a study by Kang and colleagues, 66 patients who had known or suspected renal cancer were analyzed retrospectively with a total of 90 FDG-PET scans. In this study, FDG-PET was less sensitive than CT in detecting the primary tumors and metastatic disease. (One limitation of this study, however, is that the PET images were interpreted without attenuation correction; the lack of correction limits the sensitivity of any tomographic emission imaging method).
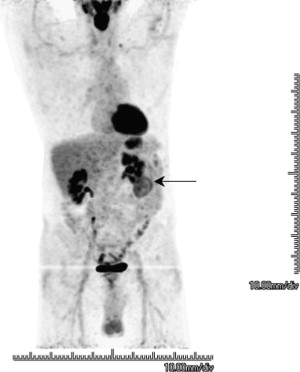
Ramdave and colleagues demonstrated comparable accuracy for FDG-PET and CT in evaluating renal masses. FDG-PET was more accurate in detecting local recurrence and metastatic disease, directly affecting the treatment in 40% of the patients. Fig. 1 shows a clear cell renal cell carcinoma.
Acetate metabolism
Acetate is taken up by cells, converted into acetyl-coenzyme A in the mitochondria, and then metabolized in the citric acid cycle and cleared in the form of CO 2 . Acetate imaging, in the form of carbon-11 (C-11)-acetate PET, has been used mostly to evaluate myocardial oxidative metabolism. C-11-acetate PET also was shown to be an accurate noninvasive technique for evaluating physiologic renal oxidative metabolism in animals, correlating with O 2 consumption and tubular sodium reabsorption.
In tumor imaging, C-11-acetate has been studied most in prostate cancer, for early differentiation between benign and malignant prostate lesions and for detecting recurrence at low levels of prostate-specific antigen. It has a limited role in the characterization of small renal masses. Shriki and colleagues first reported C-11-acetate uptake in a patient who had renal oncocytoma. In a recent study, however, Kotzerke and colleagues found no evidence of increased uptake of C-11-acetate in patients who had renal carcinoma.
Other metabolic tracers
Attempts have been made to characterize tumors using a variety of other tracers. Two are mentioned briefly here. Radioactive thymidine, long used to characterize cellular proliferation in vivo, has been studied with C-11 and, increasingly, with fluorine-18. A concordance between thymidine uptake as assessed by PET and measures of cellular proliferation including Ki-67 immunohistochemistry has been demonstrated clearly.
Radioactive choline also has been studied as a PET agent, primarily in prostate cancer. One of the attractive features of using choline as a radiotracer is that elevated choline content in tumor also is detectable by proton magnetic resonance spectroscopy, and thus the relationship between endogenous and exogenous choline can be studied by a combination of magnetic resonance spectroscopy and PET.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








