Historically, renal osteodystrophy (ROD) was a term used to describe the metabolic bone disease caused by abnormalities in mineral homeostasis resulting from kidney failure.
1 In recent years, the recognition of a complex endocrine regulation of mineral and bone metabolism, the prominent extra skeletal manifestations of chronic kidney disease (CKD), and the association between abnormalities in mineral metabolism and increased morbidity and mortality in patients with kidney failure led to formulation of a new term—chronic kidney disease-mineral and bone disorder (CKD-MBD) (
Table 77.1)
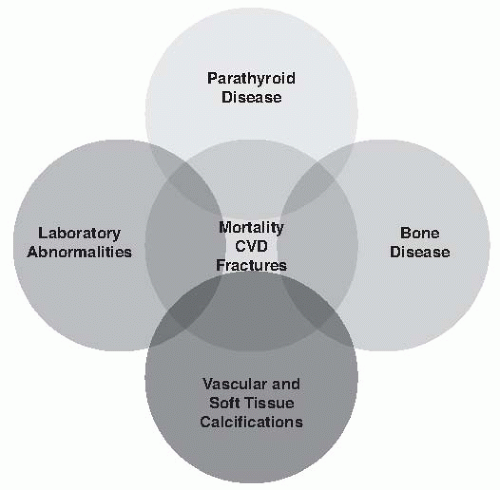
1—which describes a broader clinical syndrome, including metabolic/endocrine abnormalities, parathyroid gland dysfunction, bone disease, and unique CKD-associated cardiovascular risk factors as well as other adverse clinical outcomes, such as fractures and vascular and soft tissue calcifications (
Fig. 77.1). In this chapter we review separate components of CKD-MBD, clinical manifestations, and general principles of CKD-MBD treatment.
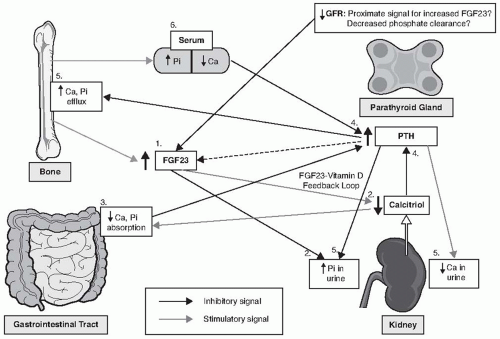
Four organ systems, including the
gut (absorption of calcium [Ca] and inorganic phosphate [Pi]),
kidneys (reabsorption and excretion of Ca, Pi, and calcitriol synthesis),
bones (interchange of Ca and Pi with extracellular pool, and FGF23 secretion), and
parathyroid gland (parathyroid hormone [PTH] secretion) are involved in regulating mineral homeostasis
2 and each play a role in the pathogenesis of CKD-MBD (
Fig. 77.2).
BONE ABNORMALITIES
Bone is central to the pathogenesis of CKD-MBD because it is: (1) a reservoir for calcium and phosphate; (2) a target for PTH, which activates PTH receptors located in osteoblasts to increase osteoblast-mediated bone resorption and to stimulate osteoclast mediated bone resorption through the release of Rank ligand; (3) a target for 1,25(OH)2D, which binds to VDR:RXR complexes to activate gene transcription in both osteoblasts and osteoclasts; and (4) the principal source of the phosphaturic and VDR hormone FGF23, which is made by osteoblasts and osteocytes.
Renal osteodystrophy (ROD) is a general term to describe the variety of skeletal histologic abnormalities that result from the changes in hormones and calcium/phosphate homeostasis in CKD.
73 The classification of ROD is based on quantitative bone histomorphometric analysis of bone biopsy that measures bone turnover (i.e., bone formation rates and resorption), mineralization of extracellular matrix, and trabecular bone volume and cortical porosity) (
Tables 77.2 and
77.3). Based on the degree of bone remodeling and mineralization abnormalities, bone biopsy diagnoses typically include osteitis fibrosa cystica (characterized by excessive PTH-mediated increases in bone formation and resorption accompanied by peritrabecular fibrosis, woven osteoid, and increased cortical porosity), osteomalacia (characterized by excess unmineralized osteoid and prolonged mineralization lag time), and adynamic bone (characterized by severely diminished bone formation and resorption). Milder forms of these abnormalities can occur and combinations of abnormal bone turnover and mineralization can occur (referred to as mixed uremic osteodystrophy). Additionally, cortical osteopenia due to excess PTH and osteoporosis due to loss of trabecular bone volume can be found in CKD and lead to increased fracture risks. Other systemic abnormalities leading to skeletal abnormalities such as β2-microglobulin amyloidosis and acidosis induced demineralization can also occur in patients with CKD.
The majority of epidemiologic data on ROD were obtained from cross-sectional analysis of bone biopsies in predialysis patients or patients on RRT; therefore, accurate data on patients with earlier stages of CKD are uncertain. The reported prevalence of ROD in CKD stage 4 and 5 ranges from 62% to 100%
5; however, given the importance of bone remodeling as a target for PTH and 1,25(OH)2D in the maintenance of calcium metabolism, virtually all patients in the late stages of CKD would be expected to have high turnover ROD, either osteitis fibrosa (OF) or mixed uremic osteodystrophy (MUO).
74 Although data are incomplete, the epidemiology of ROD appears to have changed in the last three decades, with a decline of OF and a higher prevalence of low bone remodeling states of uncertain clinical significance and etiology. Types of ROD also vary depending whether or not the patient already started RRT and on modality of RRT, with low turnover bone remodeling being the most common lesion in predialysis patients (27%—48%) and patients on peritoneal dialysis (48%-62%), whereas OF (32%-37%) and low turnover bone remodeling (32%—36%) occur with similar frequency in hemodialysis patients. Mixed disease represents about 10% to 13% of cases of ROD, and low turnover osteomalacia is present in 3% to 8% of patients. We lack diagnostic tools to accurately assess bone remodeling and mineralization, other than bone biopsy, making it difficult to determine the type and magnitude of bone abnormalities in an individual patient with CKD.
When GFR declines below 60 mL/min/m
2,
75 excess PTH and decrements in 1,25(OH)
2D are the major factors leading to abnormalities of high bone remodeling and abnormal mineralization in CKD that characterize OF and MUO. The pathogenesis of PTH and 1,25(OH)
2D alterations in CKD were discussed earlier in this chapter.
Low turnover bone disease is at the opposite end of the bone remodeling spectrum and is characterized by a diminished bone formation rate, a paucity of bone cells, an absence of fibrosis, and an abnormal bone mineralization. Adynamic bone disease (ABD) and osteomalacia are variants of low turnover bone disease in CKD. A reduction in the osteoid accumulation and number of bone remodeling sites are predominant features of ABD, which represent a primary defect in osteoblast-mediated bone formation or osteoclast-mediated bone resorption, whereas increased relative osteoid defines the presence of osteomalacia, which is a primary defect in the mineralization of extracellular matrix.
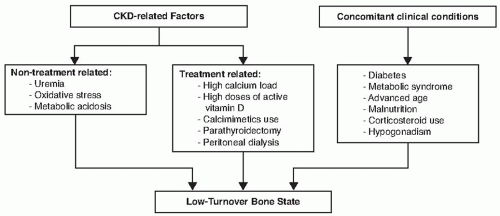
The cause of low turnover bone disease in CKD is poorly understood and it is likely to be a multifactorial condition. First reports of low turnover bone disease were osteomalacic lesions associated with aluminum toxicity; however, it was quickly recognized that low turnover bone disease can occur without aluminum accumulation in the bone. Presently, the emphasis on pathogenesis of ABD in CKD is placed on oversuppression of circulating PTH levels and concomitant skeletal resistance to PTH actions due to downregulation of PTH1R. Exposure to high Ca through the use of Ca-containing phosphate binders and dialysate with high Ca is a risk factor for ABD. Metabolic acidosis and uremia-induced oxidative stress are additional CKD-related risk factors that can induce low turnover bone disease via suppression of active vitamin D and collagen synthesis and reduction of osteoblast life span, respectively.
76 An advanced age, presence of diabetes,
hypogonadism, and a treatment with corticosteroids are also important clinical conditions associated with low turnover bone disease.
77 There is growing evidence linking ABD to the malnutrition-inflammation complex syndrome. Higher rates of ABD are reported in peritoneal dialysis patients with low albumin levels.
78 Additionally, several proinflammatory cytokines such as interleukin-1β and interleukin-6 were shown to inhibit PTH secretion in vitro.
79,
80 Therefore, the development of ABD is influenced by patient characteristics, as well as treatment options for CKD-MBD (
Fig. 77.4).
PTH is the most widely used surrogate marker of bone turnover (
Table 77.4). Although relatively low to normal iPTH levels (<50-100 pg per mL) in ESRD patients are associated with biopsy proven ABD, higher iPTH levels (>300 pg per mL) can be also be seen in patients with biopsy-proven ABD,
81 especially in African Americans.
82,
83 There are several proposed explanations of this variability of iPTH levels and ABD. First, iPTH assays and their ability to discriminate between whole PTH and its fragments differ across the studies. Some PTH fragments, such as 7-84PTH, can be actually inhibitory on bone formation and these fragments tend to accumulate in ESRD; therefore, higher PTH may not be equivalent of presence of high biointact PTH. Additionally, treatment modalities
may influence bone formation rate independently from PTH levels. Lastly, PTH is not a bone-derived marker and therefore may never be a fully accurate indicator of bone turnover. At present, it is unknown what levels of PTH are associated with ABD in patients with less severe CKD not yet on renal replacement therapy. Bone-specific alkaline phosphatase (BSAP) may be an additional useful marker of ABD. Low levels of BSAP predict ABD and BSAP correlates with bone turnover in ESRD patients treated with hemodialysis.
82
Fracture Risks in Chronic Kidney Disease
Patients with ESRD have fourfold increased risk of fractures; and the highest risk (10- to 100-fold increase) of fractures is observed in ESRD patients below age 65 as compared with age-matched individuals from the general population.
84 The risk of fractures is also augmented in early CKD.
85,
86 Vertebral and hip fractures are shown to independently increase all-cause mortality in CKD patients.
87,
88 The fracture risk in patients with low turnover bone disease remains controversial as no biopsy-proven studies are available investigating the association between adynamic bone disease and fractures in CKD patients. Because ABD is linked to PTH oversuppression, several studies demonstrated the association between relatively low to normal PTH levels and the risk of vertebral and hip fractures.
89,
90 However, in a case-control study, dialysis patients who underwent parathyroidectomy were found to have 32% lower risk for hip fractures, and 31% lower risk for any fractures as compared with matched controls.
91
Bone Disease and Vascular Calcifications