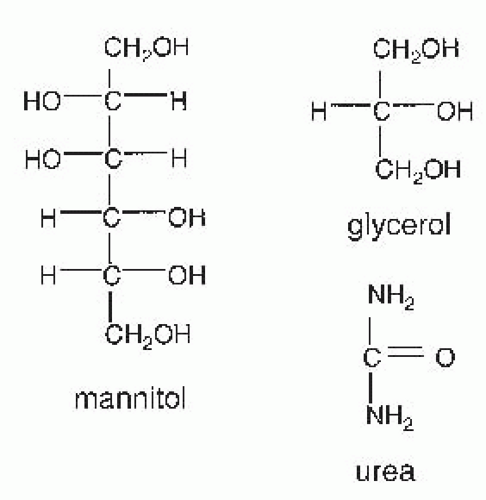
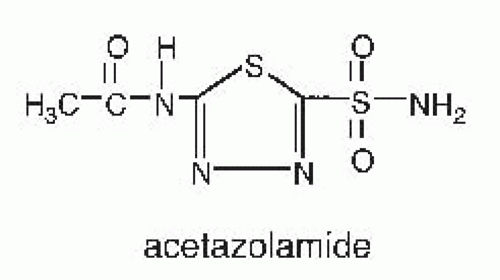
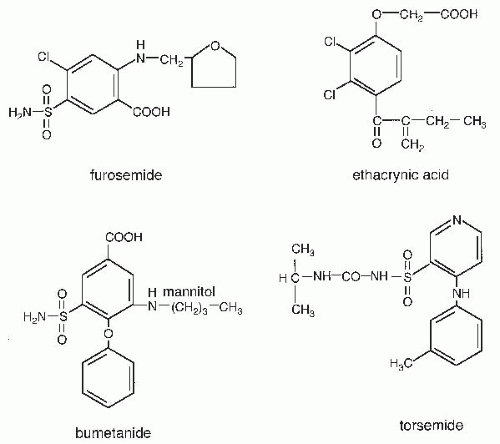
In the kidney, carbonic anhydrase inhibitors act primarily on proximal tubule cells to inhibit bicarbonate absorption.
44 Carbonic anhydrase, a metalloenzyme containing one zinc atom per molecule, is important in sodium bicarbonate reabsorption and hydrogen ion secretion by renal epithelial cells. The biochemical, morphologic, and functional properties of carbonic anhydrase have been reviewed.
45 Carbonic anhydrase isoforms (CA) can be categorized into four groups: (1) cytosolic, I, II, III, VII; (2) mitochondrial, V; (3) membrane associated IV, IX, XII, XIV; and (4) secreted, VI.
45 Carbonic anhydrases regulate cellular H ion secretion through catalyzing the formation of HCO
3 from OH and CO
2 and by binding to transporters and directly regulating activity. There are three major renal carbonic anhydrases. Type II carbonic anhydrase (CAII) is distributed widely comprising more than 95% of the overall activity in kidney and is sensitive to inhibition by sulfonamides. CAII is expressed in the cytoplasm and facilitates the secretion of H ions by catalyzing the formation of HCO
3 from OH and CO
2 (see
equation 66.3). In addition, CAII binds to the C-terminal region of NHE1 and likely regulates the transport efficiency of Na/H exchange. CAIV is bound to renal cortical membranes, comprising up to 5% of the remaining overall activity in rodent kidney, and is also sensitive to sulfonamides. Carbonic anhydrase activity at basolateral and luminal plasma membranes of proximal tubule cells and luminal membrane of intercalated cells catalyzes the dehydration of intraluminal carbonic acid generated from secreted protons. The carbonic anhydrase activity at the basolateral and luminal plasma membranes of proximal tubule cells is thought to be due in part to CAIV.
46 CAIV has been shown to also bind to the extracellular loop of NaHCO
3 transporter 1 (NBC1) regulating its transport activity.
47 Evidence for the physiologic importance for carbonic anhydrase is apparent as a deficiency of CAII leads to a renal acidification defect resulting in renal tubular acidosis. Furthermore, metabolic acidosis leads to an adaptive increase in both CAII and IV carbonic anhydrase mRNA expression in kidney
48 suggesting the importance of both carbonic anhydrase isoforms in this disorder. CAXII is also expressed in proximal tubules and collecting ducts and may contribute to the carbonic anhydrase activity in these segments.
49,
50,
51Normally the proximal tubule reabsorbs 80% of the filtered load of sodium bicarbonate and 60% of the filtered load of sodium chloride. Early studies by Pitts and micropuncture studies by DuBose and others indicated that hydrogen ion secretion is responsible for bicarbonate absorption and renal acidification. The cellular mechanism by which proximal
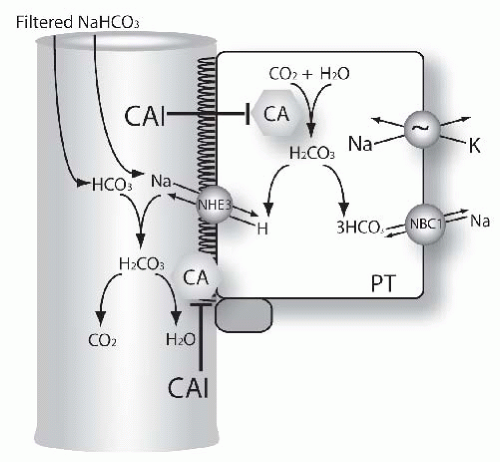
tubules reabsorb bicarbonate is depicted in
Figure 66.3. The effect of carbonic anhydrase to accelerate bicarbonate is a result of the reactions that occur in both luminal fluid and in the cell. The mechanism of carbonic anhydrase action in luminal fluid,
52 is shown here, where
E represents the carbonic anhydrase enzyme:
Luminal Fluid
Note that the addition of reactions 1 and 2 leads to the classic reaction 3. In this scheme, the enzyme is viewed as a superhydroxylator.
Luminal carbonic anhydrase prevents H from accumulating in tubule fluid, which would eventually stop all Na/H exchange. Once formed, carbon dioxide diffuses rapidly from the lumen into the cell across the apical membrane.
The mechanism by which intracellular carbonic anhydrase participates in net H+ secretion is functionally the reverse of the reactions shown previously.
Intracellular Fluid
In this case, the enzyme splits water, thereby providing an hydroxyl ion to form bicarbonate. The bicarbonate ions then exit the basolateral membrane via Na(HCO
3)
3 cotransport.
53 Thus, in the early proximal tubule, the net effect of the process described results in the isosmotic reabsorption of NaHCO
3. The lumen chloride concentration increases because water continues to be reabsorbed, thereby producing a lumen positive potential. These axial changes provide an electrochemical gradient for transport of chloride, via paracellular and transcellular pathways. The latter pathway for chloride likely involves an exchange of Cl with anions, including oxalate and formate, operating in parallel with a Na/H proton exchanger. The dual operation of these parallel exchangers results in net NaCl absorption.
54Carbonic anhydrase inhibitors act primarily on proximal tubule cells, where approximately 60% of the filtered load of sodium chloride is reabsorbed. Despite the magnitude of sodium chloride reabsorption in the proximal tubule segment, the natriuretic potency of carbonic anhydrase inhibitors is relatively weak. Several factors explain this observation. First, proximal sodium reabsorption is mediated by carbonic anhydrase-independent as well as carbonic anhydrase-dependent pathways. Second, the increased sodium delivery to distal nephron segments is largely reabsorbed by these distal nephron segments. Third, carbonic anhydrase inhibitors generate a hyperchloremic metabolic acidosis further reducing the effects of subsequent doses of carbonic anhydrase inhibitor. Metabolic acidosis also produces resistance to carbonic anhydrase action. Following the induction of metabolic acidosis, the K
i for bicarbonate absorption by membrane impermeant carbonic anhydrase inhibitors was increased by a factor of 100 to 500, suggesting that metabolic acidosis is associated with changes in the physical properties of the carbonic anhydrase protein.
55 For these reasons, carbonic anhydrase inhibitors alone are rarely used as diuretic agents.
Following carbonic anhydrase inhibitor administration, proximal tubule bicarbonate reabsorption declines between 35% and 85%. Additional sites of action of carbonic anhydrase inhibitors include proximal straight tubule or loop of Henle, distal tubule, and the collecting and papillary collecting ducts. Yet, despite the effect of carbonic anhydrase inhibitors on proximal tubules as well as other nephron segments, compensatory reabsorption of bicarbonate at other downstream
tubular sites limits net fractional excretion of bicarbonate to ˜25% to 30%, even during acute administration.
56,
57The relative contributions of membrane-bound and intracellular components of cellular carbonic anhydrase have been examined. Both species contribute to bicarbonate absorption. The role of membrane-bound carbonic anhydrase was addressed in studies that employed carbonic anhydrase inhibitors with different abilities to penetrate proximal tubule cell membranes. Benzolamide is charged at normal pH and does not penetrate cell membranes well, whereas acetazolamide enters the cell relatively easily.
58 Proximal tubular perfusion of benzolamide inhibits bicarbonate reabsorption by 90%
59 indicating that luminal carbonic anhydrase inhibition contributes importantly to bicarbonate absorption. Inhibition of luminal carbonic anhydrase causes lumen pH to decrease because of the continued secretion of hydrogen ions into the tubule lumen.
59 The conclusion that tubular fluid is in direct contact with membrane carbonic anhydrase was substantiated by the use of dextran-bound carbonic anhydrase inhibitors.
60,
61 In proximal tubules perfused in vivo, Lucci et al. determined that dextran-bound inhibitors, which inhibit only luminal carbonic anhydrase, decreased proximal tubule bicarbonate absorption by approximately 80% and reduced lumen pH.
61Although these studies establish the importance of luminal carbonic anhydrase, they also support an important role for intracellular and basolateral carbonic anhydrase. Both acetazolamide and benzolamide inhibit proximal tubule bicarbonate reabsorption to a similar degree yet they produce opposite effects on tubule fluid pH, suggesting that intracellular carbonic anhydrase contributes to proximal tubule luminal acidification. Furthermore, inherited deficiency of the predominant renal carbonic anhydrase, CAII, causes proximal renal tubular acidosis.
62The expression of carbonic anhydrase in the basolateral membrane of proximal tubule cells suggests that this membrane-bound enzyme also has an important role in basolateral bicarbonate transport. Although it is well known that carbonic anhydrase inhibitors inhibit intracellular generation of substrate for the transporter,
63,
64 the direct interaction between CAIV and NBC1, the sodium/bicarbonate cotransporter,
47 suggests the possibility that carbonic anhydrase inhibitors may also directly regulate anion transport activity. Functional studies using an impermeant carbonic anhydrase inhibitor,
p-fluorobenzyl-aminobenzamide, that is 1% as permeable as acetazolamide, demonstrated the importance of basolateral membrane-bound carbonic anhydrase.
p-Fluorobenzyl-aminobenzamide reduced fluid and bicarbonate absorption when applied to the basolateral membrane of rabbit proximal tubules perfused in vitro.
50In the collecting duct, carbonic anhydrase facilitates acid secretion that is mediated by a vacuolar H adenosine triphosphatase (H-ATPase)
65 and a P-type gastric H-K-ATPase.
66,
67,
68 Luminal administration of acetazolamide produced an acid disequilibrium pH in the outer medullary collecting duct suggesting the contribution of luminal carbonic anhydrase.
69Using a membrane-impermeant carbonic anhydrase inhibitor (F-3500; aminobenzamide coupled to a nontoxic polymer polyoxyethylene), bicarbonate absorption was reduced confirming the presence of carbonic anhydrase in the luminal membrane of the outer medullary collecting duct.
55 The K
i for inhibition of bicarbonate absorption was 5 µM, consistent with the inhibition of CAIV.
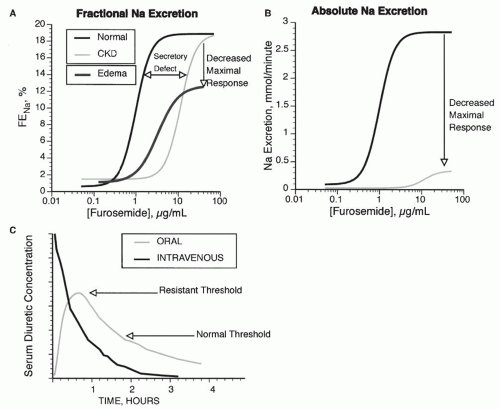
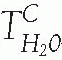 ). During maximal loop diuretic action, the urinary Na concentration is usually between 75 to 100 mM.98 Because urinary K concentrations during furosemide-induced natriuresis remain low, this means that the clearance of electrolyte free water (CH2Oe) is increased when loop diuretics are administered during conditions of water diuresis or hydropenia.98 This effect of loop diuretics has been exploited to treat hyponatremia, when combined with normal or hypertonic saline.99,100
). During maximal loop diuretic action, the urinary Na concentration is usually between 75 to 100 mM.98 Because urinary K concentrations during furosemide-induced natriuresis remain low, this means that the clearance of electrolyte free water (CH2Oe) is increased when loop diuretics are administered during conditions of water diuresis or hydropenia.98 This effect of loop diuretics has been exploited to treat hyponatremia, when combined with normal or hypertonic saline.99,100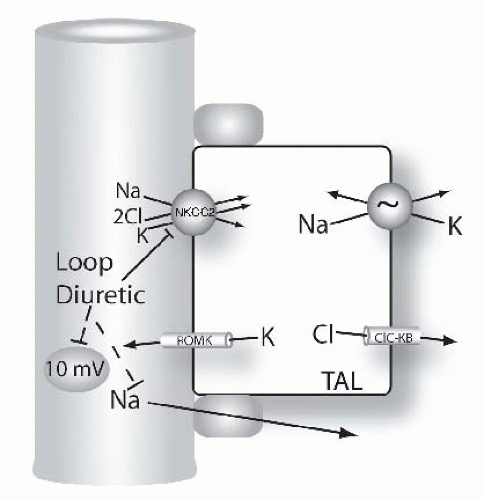
 (10%-25%)
(10%-25%) (15%-30%)
(15%-30%) (6%)
(6%) (5%-10%)
(5%-10%) (10%-20%)
(10%-20%) (>20%)
(>20%) (6%)
(6%) (4%)
(4%) (60%)
(60%) (>20%)
(>20%) or ⇔ (<5%)
or ⇔ (<5%) (<5%)
(<5%) (30%)
(30%) (40%)
(40%) (60%-100%)
(60%-100%) (>20%)
(>20%) (>20%)
(>20%) (>20%)
(>20%) (6%-11%)
(6%-11%) (10%)
(10%) (200%)
(200%) (>20%)
(>20%)
 (5-10%)
(5-10%) (1%-5%)
(1%-5%) (6%)
(6%) (8%)
(8%)
 indicates that the drug increases excretion;
indicates that the drug increases excretion;  indicates that the drug decreases excretion; ⇔ indicates that the drug has little or no direct effect on excretion. During chronic treatment, effects often wane (Na excretion), may increase (K excretion during distal convoluted tubule diuretic treatment), or may reverse as with uric acid (not shown).
indicates that the drug decreases excretion; ⇔ indicates that the drug has little or no direct effect on excretion. During chronic treatment, effects often wane (Na excretion), may increase (K excretion during distal convoluted tubule diuretic treatment), or may reverse as with uric acid (not shown).