Chapter 36 Malignant Biliary Obstruction
Distal
Malignant strictures of the pancreaticobiliary tree are often difficult to diagnose and treat. They present late and hence these factors contribute to the poor prognosis of these tumors. Jaundice is the most common presenting sign and symptom of malignant biliary obstruction. The causes of distal malignant biliary obstruction include pancreatic cancer, carcinoma of the ampulla of Vater, gallbladder cancer, distal cholangiocarcinoma and metastatic disease that involves the head of the pancreas or the common bile duct (CBD) (Table 36.1). Obstructive jaundice often presents in the context of advanced disease and negatively affects the quality of life. This chapter discusses the role of endoscopic retrograde cholangiopancreatography (ERCP) in the management of malignant distal biliary obstruction. Management of biliary obstruction in the setting of proximal biliary obstruction is discussed separately in Chapter 37.
Epidemiology
The most common cause of malignant distal biliary obstruction is pancreatic cancer. In the United States an estimated 43,920 new cases of pancreatic cancer will occur in 2012 with an estimated 37,390 deaths expected to occur from this condition.1 The incidence rate of pancreatic cancer has been increasing by 1.5% per year since 2004, with an increase in mortality rates of 0.4% per year during this period. By 2012 estimates, pancreatic cancer ranks among the top 10 newly diagnosed cancers in the United States in both sexes, and it ranks fourth in cancer-related deaths in both sexes.1 Although globally pancreatic cancer is considered a rare form of cancer (2.5% of all cancers), it still ranks among the top 10 cancer-related deaths, mainly because of its exceptionally high mortality. Because the incidence of pancreatic cancer varies greatly across regions, lifestyle and environmental factors have been suggested to play a role in its etiopathogenesis. Advancing age is a recognized risk factor for pancreatic cancer. The median age at diagnosis of pancreatic cancer in the United States is 72 years. About 5% to 10% of patients develop pancreatic cancer before they are age 50 years, but this group is more likely to include those with predisposing genetic disorders or those who have undergone treatments for cancer such as previous radiation.2,3 Cigarette smoking has been strongly associated with pancreatic cancer and the risk increases by 70% to 100% over nonsmokers. Heavy consumption of alcohol (three or more drinks per day or greater than or equal to 30 to 40 g of alcohol per day) is associated with a 22% increase in the risk of pancreatic cancer.4 Underlying chronic pancreatitis, although rare, increases the risk for developing pancreatic cancer. Other implicated risk factors for pancreatic cancer include vitamin D and ultraviolet B (UVB) radiation, occupational exposure, and obesity. A family history of pancreatic cancer is associated with a twofold increase in the pancreatic cancer risk.5 Genetic factors that increase the risk of pancreatic cancer include familial atypical multiple mole melanoma (FAMMM) syndrome, hereditary pancreatitis, Peutz-Jeghers syndrome, familial pancreatic cancer, cystic fibrosis, familial adenomatous polyposis, and hereditary nonpolyposis colorectal cancer (HNPCC) syndrome.
Cholangiocarcinoma can arise either within the liver (intrahepatic) or in the extrahepatic bile ducts (extrahepatic). Globally, cholangiocarcinoma is the second most common primary hepatic malignancy.6 Recent epidemiologic studies show that the incidence and mortality rates of intrahepatic cholangiocarcinoma are increasing while those of extrahepatic cholangiocarcinoma are falling globally. The peak age for cholangiocarcinoma is the seventh decade and the incidence is higher in men. Most cases are sporadic and only in a minority of patients are specific risk factors identified. Factors that result in chronic inflammation of the bile duct are often implicated. Some of these risk factors include advanced age, male gender, and underlying conditions such as primary sclerosing cholangitis (PSC), fibropolycystic liver disease, Caroli disease, choledochal cysts, HNPCC, and bile duct adenomas. PSC particularly is associated with earlier onset of cholangiocarcinoma. Rarely, cholangiocarcinoma has been associated with diabetes, obesity, viral hepatitis (hepatitis C and possibly hepatitis B), lifestyle factors such as smoking and alcohol use, and exposure to toxins such as asbestos, nitrosamines, and thorotrast. In Asia, especially Thailand and China, infestation with liver flukes Clonorchis sinensis and Opisthorchis viverrini is strongly associated with cholangiocarcinoma.
In the United States gallbladder cancer is the fifth most common gastrointestinal cancer and the most common cancer involving the biliary tract. Among Southwestern Native Americans and Mexican Americans, gallbladder cancer is the most common gastrointestinal malignancy.7
Cholelithiasis is a well-described, and the strongest, risk factor for gallbladder cancer. A majority of patients will have incidental detection of gallbladder cancer when they undergo investigation for cholelithiasis. Cancer will be detected in less than 1% of cases.6
Other risk factors include advanced age, female gender, and specific geographical areas. In South American countries such as Chile, Ecuador, and Bolivia and in Asian countries such as India, Pakistan, Japan, and Korea, the incidence of gallbladder cancer is higher. Underlying gallbladder conditions such as porcelain gallbladder, gallbladder polyps, congenital biliary cysts, and abnormal pancreaticobiliary duct junction are associated with higher risk of cancer of the gallbladder.6 Smoking and obesity have also been implicated, although the evidence linking them to gallbladder cancer is weak. The potential risk factors of pancreaticobiliary malignancies are summarized in Table 36.2.
Clinical Features
The most common clinical presentation of pancreatic and biliary malignancies includes painless jaundice, weight loss, and anorexia. The elevation in bilirubin associated with biliary obstruction leads to scleral icterus, pale stools, dark urine, pruritus, and nausea. Advanced forms of pancreatic cancer can present with epigastric pain with radiation to the back suggesting pancreatic duct obstruction and infiltration of retroperitoneal structures, palpable gallbladder, dyspepsia that may be secondary to gastric outlet obstruction, new-onset glucose intolerance or diabetes, and acute pancreatitis. Cholangiocarcinoma presents with abdominal pain mostly in the right upper quadrant, jaundice, pruritus, and weight loss. Risk factors should be sought during history taking. For pancreatic cancer, these include a history of tobacco smoking and smokeless tobacco usage, family history of pancreatic cancer, personal history of diabetes, pancreatitis, and obesity. For cholangiocarcinoma, a history of previously known conditions should be obtained, such as inflammatory bowel disease, PSC, cholelithiasis, choledochal cysts, Caroli disease, HNPCC, human immunodeficiency virus (HIV), and diabetes. History of obesity, smoking, alcohol use, and exposure to toxic substances such as asbestos and nitrosamines should also be obtained. A complete physical exam should include identification of icterus and palpation to identify organomegaly, including liver, gallbladder, and lymph nodes. Migratory thrombophlebitis can be recognized via positive Trousseau sign or swollen visible blood vessels that resolve and then appear at another area of the body. The symptoms and physical exam findings of these conditions are summarized in Table 36.3. Laboratory tests should include total bilirubin, alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and tumor markers such as cancer antigen (CA) 19-9 and carcinoembryonic antigen (CEA). Further testing with advanced imaging studies should be based on the initial suspicion of pancreaticobiliary malignancy from the history, physical exam, and initial laboratory test results.
Differential Diagnosis of Distal Biliary Malignancies and Imaging Techniques
Ampullary Carcinoma
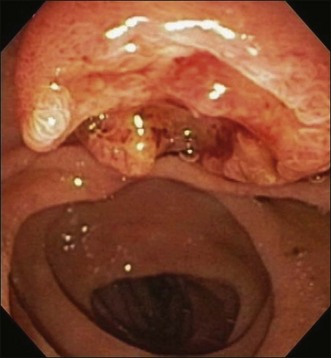
Ampullary carcinoma often presents with obstructive jaundice and it is suspected if imaging studies detect dilation of the pancreaticobiliary ducts. Ultrasound is not very sensitive to detect ampullary carcinoma due to the small size of the tumor. In addition, overlying bowel gas limits the views of the retroperitoneal structures. Thus ultrasound relies more on indirect evidence such as dilated bile ducts. Computed tomography (CT) scan provides more accuracy than ultrasound in this regard but still a substantial number of lesions could be missed. Magnetic resonance cholangiopancreatography (MRCP) is another noninvasive technique and is superior to CT scan in detecting biliary obstruction. However, MRCP cannot differentiate between tumors and other benign causes of ampullary obstruction such as stones or benign strictures.8 ERCP can be both diagnostic by detecting an ampullary mass and providing tissue samples (Fig. 36.1) and therapeutic for relieving obstructive jaundice. EUS and ERCP are comparable in terms of detecting ampullary cancers. EUS is the most accurate imaging modality to provide information about the local tumor staging of ampullary neoplasms.9,10 When cure appears feasible through surgery, the choice of surgery such as local resection versus a more radical surgery such as pancreaticoduodenectomy is determined by the staging information obtained from the noninvasive techniques discussed above.
Pancreatic Cancer
The pancreatic malignancies that cause distal biliary obstruction involve the head of the pancreas, although large lesions involving other areas of the pancreas can also obstruct the bile duct. The workup usually begins with a combination of imaging studies. These tools include transabdominal ultrasound, CT scan, magnetic resonance imaging (MRI), ERCP, and EUS. These imaging tools enable cancer diagnosis and staging, determine tumor resectability, and aid in providing a histopathologic diagnosis of cancer. Transabdominal ultrasound with and without contrast has a diagnostic accuracy of about 82% to 86% for diagnosing pancreatic head malignancies.11 Recent advances in ultrasound technology, including incorporation of Doppler studies, angiography, and three-dimensional ultrasound imaging, are expected to improve the diagnostic yield even more.
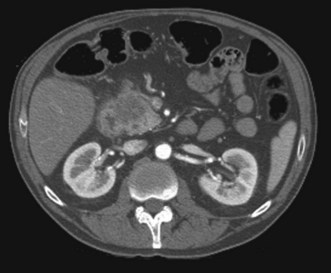
CT scanning improves tumor detection and provides information about the local invasion by the tumor and invasion of the vascular structures. Dynamic contrast-enhanced multidetector CT scan has significantly improved pancreatic cancer imaging, with an ability to provide enhanced three-dimensional reconstructions of pancreatic tumors and their vascular involvement (Fig. 36.2). The sensitivity of multidetector CT ranges from 63% to 92% and this is currently the preferred modality for preoperative staging and assessment of resectability of patients with pancreatic malignancy.12
MRI at a field strength of 1.5 Tesla (T) with either gadolinium- or mangafodipir-enhanced sequences is equally useful in detecting pancreatic cancers. The types of MRI imaging available to evaluate the pancreas include conventional MRI, MRCP, and magnetic resonance angiography. A recent study that compared multidetector CT scan and MRI reported comparable diagnostic accuracies for tumor identification and resectability for the two techniques.13 Accuracy of CT scan and MRI has been shown to be comparable for characterization of small pancreatic tumors less than or equal to 2 cm.14 A recent study that compared multidetector (64-detector row) CT scan to gadobenate dimeglumine–enhanced 3.0 T MRI imaging (compared to previously used 1.5 T MRI imaging) also showed similar sensitivities and specificities for the two techniques in detecting pancreatic cancer.15 While in routine practice it is not uncommon to use both CT and MRI to detect pancreatic cancer, MRI is selectively useful in specific cases where the patient cannot be administered iodinated contrast material due to either contrast allergy or renal insufficiency.
A systematic review that included 11 studies and 678 patients concluded that EUS had a higher sensitivity compared to CT scan in detecting pancreatic tumors.16 A study that evaluated the accuracy of EUS, dynamic CT, and MRI in detecting pancreatic tumors less than 3 cm in diameter found a sensitivity of 93% for EUS, 53% for CT, and 67% for MRI, thus highlighting the importance of EUS in detecting tumors less than 3 cm in diameter.17 However, it must be noted that multidetector CT scanning was introduced after most of the above studies were published. In addition to tumor characteristics, multidetector CT scanning provides a more complete assessment of vascular invasion compared with conventional CT scans. Thus further studies are awaited for comparing EUS, CT, and MRI using the current technology.
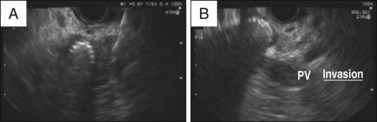
The staging of pancreatic cancer follows the Tumor, Node, Metastasis (TNM) staging proposed by the American Joint Committee on Cancer (AJCC). The TNM staging system has undergone periodic changes. With the current TNM staging system, recent studies have reported that EUS is superior to multidetector CT in terms of T-staging and has overall lower overstaging accuracy when compared with CT and MRI.18,19 With regard to N-staging, the studies that have compared CT and EUS have not conclusively established the superiority of one technology over the other.18,20 For M-staging, the advantage of multidetector CT scan over EUS rests with the fact that, in addition to local spread, CT scan also provides information about distal metastasis. Thus CT scan currently is more commonly used for the initial staging of pancreatic cancer. EUS becomes a valuable staging tool in situations where CT scan provides equivocal results about surrounding lymph nodes or when small solid tumors less than 3 cm are detected on CT scan.12 With advances in cross-sectional imaging and EUS, currently ERCP has a minimal role in the diagnosis and staging of pancreatic cancer. However, certain signs observed during ERCP should alert the clinician to the possibility of pancreatic cancer. The finding of combined dilation of the bile duct and the pancreatic duct (double-duct sign) occurs in the context of obstruction by a mass in the head of the pancreas and once was considered pathognomonic of this condition. Similarly, findings such as abrupt cutoff of the pancreatic duct or a single long stricture (>1 cm) of the pancreatic duct, although not specific to pancreatic cancer, should raise suspicion for this condition.
If the initial imaging studies are suggestive of pancreatic malignancy and the patient is an appropriate operative candidate, it is reasonable to refer the patient for surgery for resection. Tissue diagnosis can then be obtained from the surgical specimen. Due to late presentation of this disease, only a small proportion of patients are considered to be surgical candidates (15% to 20%). More commonly, cytologic diagnosis of pancreatic cancer outside of surgery is performed by FNA (ultrasonography-guided, CT-guided, or EUS-guided) and brush cytology (obtained during ERCP). The EUS-guided FNA has a sensitivity of about 98%, a specificity of about 85%, and a positive predictive value that approaches 100% but has a low negative predictive value.21 Thus a negative EUS-FNA result cannot reliably rule out cancer. A recent randomized trial that compared EUS-guided FNA with CT-guided FNA found no significant differences in the sensitivities of the two techniques.22 However, EUS is the test of choice when the tumor size is less than 3 cm (Fig. 36.3).
The adverse events of EUS-FNA include bleeding, pancreatitis, perforation, and tumor seeding. In a prospective study that included 355 patients with solid pancreatic tumor, the overall adverse event rate was 2.54% (3 cases of pancreatitis), with 1.9% of patients requiring hospitalization.23 Even in the setting of centers with less experienced operators, the adverse event rates with EUS-FNA were reported to be as low as 1.1%.24
There have been three reports of tumor seeding as a result of EUS-FNA, with seeding to the gastrointestinal wall.25 There have also been reports of development of peritoneal carcinomatosis. However, when compared with percutaneous biopsy guided by ultrasonography or CT scan, the risk of peritoneal tumor seeding with EUS-FNA has been reported to be less common (16.3% versus 2.2%).26
In addition, cytology specimens can be taken at the time of biliary decompression at the time of ERCP. Brush cytology has a sensitivity that ranges from 30% to 60%. This sensitivity can be augmented by obtaining multiple brush cytology samples before and after stricture dilation and by sending the entire brush for analysis.27,28 Brush cytology has a high positive predictive value but poor negative predictive value. If brush cytology is nonconclusive, cholangioscopy-guided biopsies of the bile duct can be performed and have up to 82% accuracy of differentiating between benign and malignant strictures.29
Cholangiocarcinoma
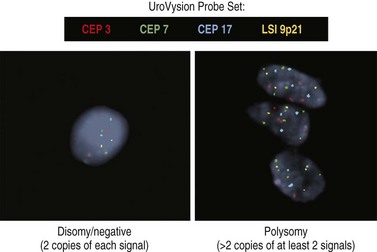
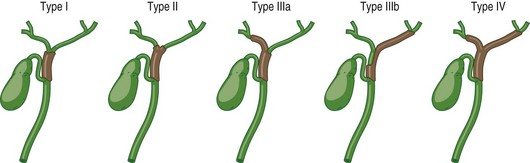
Intrahepatic cholangiocarcinoma may present as one or more mass lesions on imaging studies. Hilar and distal cholangiocarcinoma is suspected in patients presenting with biliary obstruction, right upper quadrant abdominal pain, and cholangitis. Noninvasive biliary visualization with MRCP is the radiologic modality of choice to determine the extent of the disease. In addition, MRCP may provide a road map to biliary stents.30 However, if biliary obstruction is present or if suspicious lesions need to be sampled, either ERCP with brush cytology or ERCP and cholangioscopy with bile duct biopsies may be required. Newer cytologic techniques such as digital image analysis (DIA) and fluorescence in situ hybridization (FISH) have been incorporated into the cytologic evaluation of bile duct brushings to enhance sensitivity of cytology (Fig. 36.4; see also Chapter 38).31,32 Both techniques identify aneuploidy, which is a hallmark of chromosomal instability and cancer. The combination of DIA and FISH offers the highest sensitivity for the diagnosis of malignant biliary strictures in patients both with and without PSC in a single-center experience.33 EUS may be useful in determining the extent of disease and sampling regional lymph nodes. Surgical resectability and type of surgery can be aided by the Bismuth-Corlette classification of hilar cholangiocarcinoma (Fig. 36.5). While surgical resection is the mainstay of curative treatment for cholangiocarcinoma when PSC is absent, surgery is not recommended in patients with PSC due to multifocality of the disease. There is a poor impact on overall disease mortality after surgery. In patients with PSC with early cholangiocarcinoma, liver transplantation is the preferred definitive surgery.33 Distal bile duct cancers require concomitant pancreatoduodenectomy. Factors that can affect surgical decision include poor performance status, presence of cirrhosis, and other comorbidities. Five-year survival for distal bile cancers approximates 37%.34
Metastatic Disease
The cancers that metastasize and cause malignant biliary tract obstruction in order of frequency are gastric, colon, breast, and lung cancers. Others include renal cell carcinoma, melanoma, and hepatocellular cancer. Occasionally malignant lymphadenopathy can cause malignant biliary obstruction (Table 36.1). These lesions may cause either extrinsic or intrinsic compression of the bile duct. Often a primary source of cancer is known, making diagnosis easy. In other instances the lesion may be discovered when endoscopic or surgical management of biliary obstruction is attempted. Imaging modalities such as CT scan and MRI may be useful in determining the level of the obstruction and may help determine the best management option for obstructive jaundice. Due to advanced disease, except in very limited cases, palliation becomes the only option available to these patients.
An Approach to the Management of Patients with Distal Biliary Malignancies
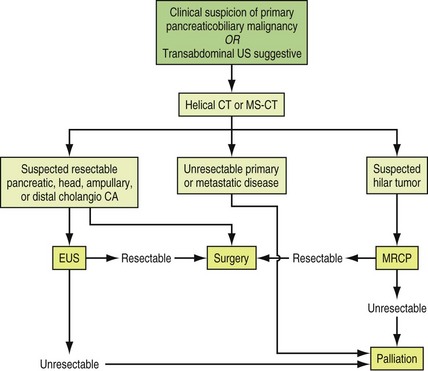
The initial approach to pancreaticobiliary malignancy is to establish a diagnosis and stage the disease. CT and MRI scans may establish the site of malignancy, vascular involvement, and the presence and absence of metastatic disease. Tissue may be procured for histopathologic assessment by EUS if the lesion is within the pancreas, within the distal CBD, or within a surrounding enlarged lymph node. ERCP with brush cytology or biopsy may reveal the diagnosis. Rarely, given the strong suspicion of cancer from imaging studies and fairly high accuracy of staging offered by these imaging modalities, a patient is referred for surgery and tissue diagnosis, and staging may be achieved during surgery. Staging is critical to determining whether or not curative surgery is possible. The management options for obstructive jaundice may also vary according to the level of biliary obstruction. If the level of obstruction is distal, endoscopic drainage or other surgical options such as choledochoduodenostomy or choledochojejunostomy may be considered. An algorithm proposed by the American Society for Gastrointestinal Endoscopy for the diagnosis and management of patients with suspicion for pancreaticobiliary malignancies is shown in Fig. 36.6.
Curative Surgery
Despite significant advances in the field of diagnosis and treatment, pancreaticobiliary malignancy is still associated with poor overall prognosis. At the time of diagnosis only 15% to 20% of patients are considered candidates for curative resection.35 The reason for categorizing a patient as a nonsurgical candidate may be because of either advanced disease or poor performance status due to comorbid conditions. For cancer that involves the head of the pancreas, the curative surgical procedure is a Whipple procedure (pancreaticoduodenectomy), which involves removal of the head of the pancreas, gallbladder, CBD, duodenum, antrum of the stomach, and the proximal part of the jejunum. In a modified Whipple procedure termed pylorus-preserving pancreaticoduodenectomy, the stomach is retained, reducing problems with bile reflux, dumping, and marginal ulceration. Both procedures have been found to be comparable in terms of mortality, morbidity, and overall survival.36 The surgical option for patients with distal cholangiocarcinoma is a pylorus-preserving pancreaticoduodenectomy, bile duct resection, and hepatobiliary resection, with 5-year survival ranging from 20% to 30%.37,38 For ampullary cancers, curative surgery is performed via either the classic Whipple procedure or a pylorus-preserving pancreaticoduodenectomy with a 5-year survival rate reported to be 37.9%.39 If a patient with pancreaticobiliary malignancy is found to have a resectable tumor after initial preoperative, noninvasive diagnostic workup through imaging studies, he or she should undergo diagnostic laparoscopy with peritoneal washings for cytology or exploratory laparoscopy to determine resectability. Palliation should be considered for patients who are determined not to be candidates for curative surgery.
Palliation
The most common symptoms that require palliation in patients with pancreaticobiliary malignancy are obstructive jaundice, pain, and duodenal obstruction. Palliation of jaundice stemming from distal malignant biliary obstruction is accomplished by one of three means: endoscopic stenting, percutaneous transhepatic biliary drainage (PTBD), or surgical biliary bypass (Table 36.4).
Table 36.4 Techniques of Palliative Biliary Drainage
| Type of Intervention | Type of Procedure | Type of Drainage |
|---|---|---|
| Surgery | Choledochojejunostomy Choledochoduodenostomy Cholecystojejunostomy Cholecystogastrostomy Cholecystoduodenostomy Hepaticojejunostomy | Internal |
| Percutaneous (image-guided) | Percutaneous internal biliary drainage Percutaneous external biliary drainage Percutaneous external-internal biliary drainage | Internal, external, or both |
| Endoscopic | Transpapillary biliary drainage Nasobiliary drainage | Internal |
Endoscopic Stenting
In patients with malignant biliary obstruction, relieving obstructive jaundice through palliative biliary decompression has shown to improve the quality of life.40 The role of ERCP in biliary decompression is discussed below. Technical aspects of endoscopic biliary stenting and the equipment are discussed in detail in Chapters 21, 22, and 23.
Background
The earliest study reporting endoscopic biliary stenting was in 1979, reported by Soehendra, who placed plastic stents in inoperable cancer patients.41 The instruments available at the time had small working channels, limiting the size of the stents to 7 Fr. The need for larger diameter stents was quickly recognized given the high incidence of obstruction and recurrent cholangitis in these patients. Therefore side-viewing endoscopes that allowed insertion of stents with diameters ranging from 7 to 12 Fr were developed. The placement of large plastic stents improved patency to an average of 3 to 4 months. It has been demonstrated that inserting biliary stents with diameters beyond 10 Fr, to 11.5 Fr, is technically more difficult and does not offer significant improvement in stent patency. As a means to overcome the limitations of size and therefore stent patency, self-expandable metal stents (SEMS) were developed. The earliest studies reporting their use to relieve biliary obstruction were published in 1989.42,43
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree