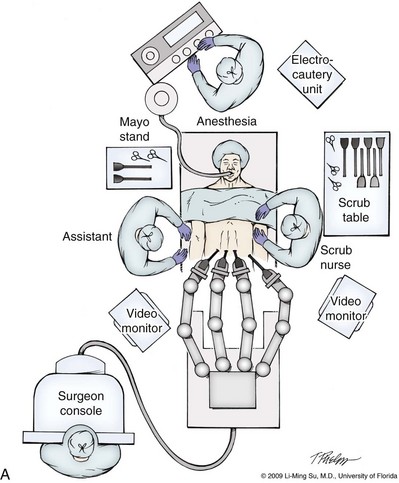
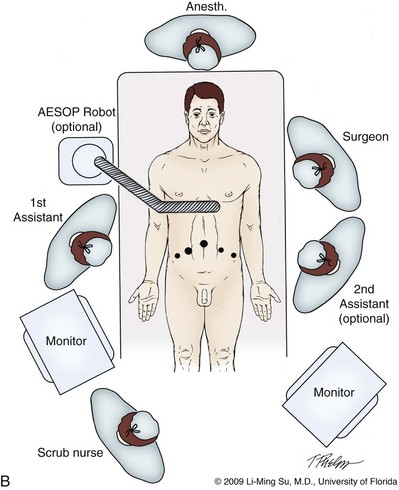
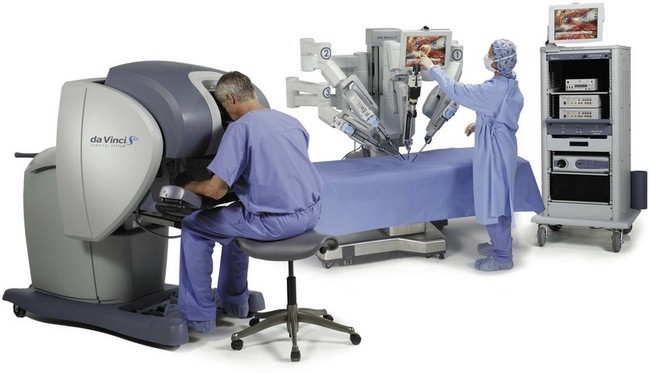
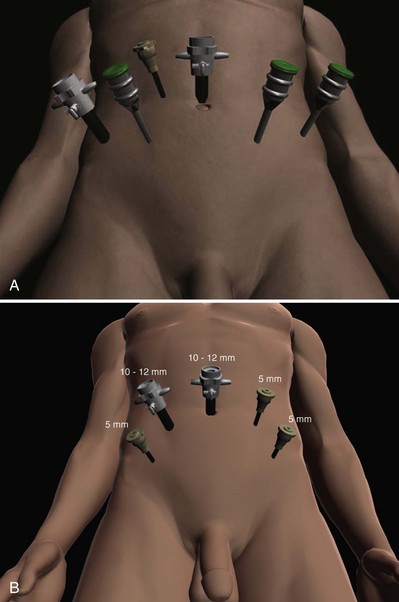
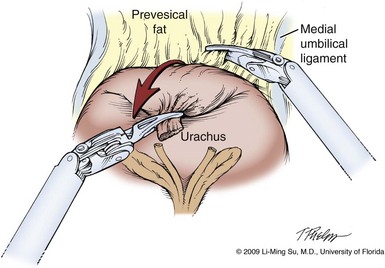
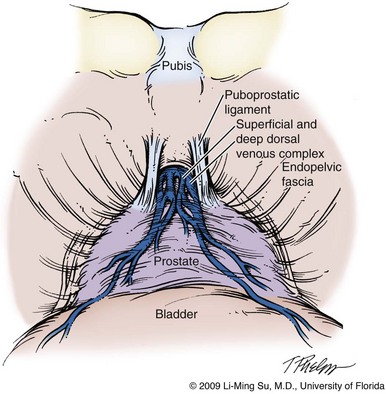
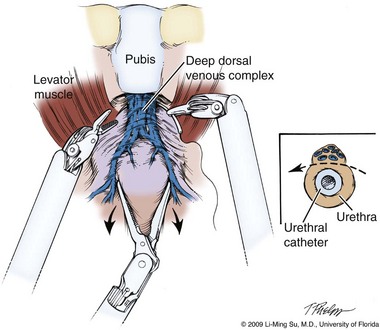
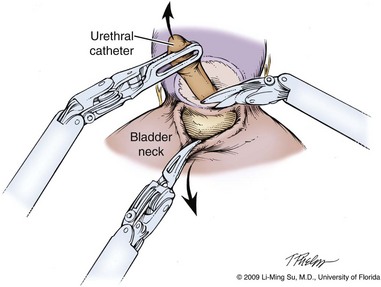
Li-Ming Su, MD, Joseph A. Smith, Jr., MD Over a century has passed since Hugh Hampton Young (1905) performed the first open prostatectomy for carcinoma through a perineal approach. In 1947, Millin was the first to describe the retropubic approach to radical prostatectomy. In the late 1970s and early 1980s several detailed anatomic studies performed in fetal and adult cadavers provided important insights into the periprostatic anatomy, especially that of the dorsal vein complex (DVC; Reiner and Walsh, 1979), the neurovascular bundle (NVB; Walsh and Donker, 1982), and the striated urethral sphincter (Oelrich, 1980). These observations provided a more anatomic approach to radical prostatectomy with a consequent reduction in operative morbidity. Subsequently, anatomic, nerve-sparing radical prostatectomy has maintained a cardinal role in the management of localized prostate cancer for more than 2 decades. Schuessler and colleagues (1997) performed the first successful laparoscopic radical prostatectomy (LRP) in 1997. In their series of nine patients, operative duration was lengthy (8 to 11 hours) and the length of stay was 7.3 days on average. Although the authors concluded that cure rates with LRP may be comparable with open surgery, they could not define any significant advantages. As a result, LRP was not immediately widely adopted in the field of urology. Advances in task-specific surgical instrumentation, optics, digital video equipment, and computer and robotic technology opened a new frontier for minimally invasive laparoscopic prostatectomy. These advances led urologists to revisit LRP, spearheaded by two centers in France that reported on their techniques and early results (Abbou et al, 2000; Guillonneau and Vallancien, 2000). Their stepwise approach to LRP proved to be both reproducible and teachable, although the learning curve remained challenging. Operative times were in a more acceptable 4- to 5-hour range with reported overall positive margin rates of 15% to 28%. Both groups had good continence and potency rates. This work rekindled worldwide interest in LRP, and in the ensuing years surgeons at a number of centers throughout the world acquired the skills and experience to perform LRP. However, advanced laparoscopic skills are necessary to perform a proficient LRP, especially for suturing of the vesicourethral anastomosis. Morbidly obese patients pose additional challenges due to the potential respiratory compromise encountered when placing these patients in a steep Trendelenburg position, as well as the relatively limited working space and limitations of trocar size and instrumentation length, especially with LRP. Patients with large prostate volumes (e.g., >70 g) are often met with longer operative times, blood loss, and hospital stay than those with smaller glands; however, long-term urinary outcomes appear comparable (Levinson et al, 2008, 2009; Link et al, 2008). Salvage surgery after failure of primary treatment (e.g., radiation, brachytherapy, cryotherapy, high-intensity focused ultrasound) has been successfully reported in properly selected patients but should be approached with caution due to the attendant risks and complications (Kaouk, 2008; Boris et al, 2009). Due to the effects of prior local radiotherapy or ablation, the tissue planes surrounding the prostate and especially between the posterior prostate and anterior rectum are often fibrotic and obliterated, increasing the risk of inadvertent entry into the rectum during salvage surgery. As a result, patients undergoing salvage prostatectomy need to be counseled on the potential risk of rectal injury in addition to the higher incidence of impotence and incontinence as compared with primary surgery. It is strongly advised that these more complex patient scenarios be avoided in a surgeon’s early experience with LRP and RALP; however, these patient features are not by themselves absolute contraindications for a minimally invasive approach to prostatectomy (Brown et al, 2005a; Erdogru et al, 2005; Singh et al, 2005; Stolzenburg et al, 2005). Instrumentation required for LRP and RALP is dependent on the chosen approach and model of da Vinci system being used (i.e., three- vs. four-arm robot) in the case of RALP. A list of suggested instrumentation for LRP and RALP are listed in Table 103–1. For LRP, the AESOP 3000 robotic arm (Intuitive Surgical, Inc., Sunnyvale, CA) may be used to stabilize and control the laparoscopic lens and camera by hand-held remote control, voice activation, or foot pedal control. Alternatively, a surgical assistant can be used for this purpose. During RALP, the use of the da Vinci S or Si HD system allows the surgeon to control a total of four robotic arms with one being the endoscope as compared with only three robotic arms with the first-generation standard robotic platform. The surgeon generally begins the operation by using a 0-degree stereo endoscope and controlling a grasping forceps in the left robotic arm (such as the Maryland curved bipolar forceps or plasma kinetic dissector) and the curved monopolar scissors in the right robotic arm. The fourth robotic arm controls the ProGrasp forceps (Intuitive Surgical, Inc., Sunnyvale, CA), a large atraumatic blunt grasper for retraction and exposure of tissues. The surgeons then toggle between control of any two of the three working robotic arms at any given time to allow for greater autonomy and to achieve optimal exposure and dissection. Table 103–1 Suggested Instrumentation for LRP and RALP LRP, laparoscopic radical prostatectomy; RALP, robotic-assisted laparoscopic prostatectomy. LRP and RALP require that the surgical team including the scrub technician, circulating nurse, and surgical assistant(s) be fully trained and skilled in the instrumentation, operative setup, and technical steps of these minimally invasive techniques. Only one skilled assistant is generally required for these procedures, but a second assistant may be used if available to provide retraction of tissues. The scrub technician is an integral part of the operative team and must be versed in the wide array of laparoscopic and robotic instruments that may be used to accomplish this procedure. For RALP, it is important for the tableside assistant to have adequate training in basic laparoscopy and the mechanics, setup, and troubleshooting of the robotic system. Typical operating room equipment and setup for RALP and LRP are shown in Figure 103–1. Both LRP and RALP require general anesthesia. Because the patient’s arms are tucked at the side and difficult to access, establishing accurate pulse oximetry, blood pressure cuff placement, and intravenous access is critical before patient positioning. The anesthesiologist must be aware of the potential consequences of CO2 insufflation and pneumoperitoneum including oliguria and hypercarbia. Prompt adjustments in minute and tidal volumes may be required by the anesthesiologist in the event of rising end-tidal CO2 levels and hypercarbia (Meininger et al, 2004). Adjustments in CO2 insufflation pressures may also be required by the surgeon to reduce the risk of continued hypercarbia. Taken together, maintaining good communication between the surgeon and the anesthesia team is important during these procedures. Most of the principles and considerations for the surgical dissection are similar regardless of whether a pure laparoscopic or robotic-assisted approach is used. For RALP, the da Vinci Surgical System is a master/slave system with three components: surgical robot (also called the patient side cart), surgeon console, and video cart (Fig. 103–2). For the purpose of this chapter and for simplicity, the technique using the four-arm da Vinci S system is described. The robot is docked at the foot of the operating table between the patient’s legs. The tableside assistant is responsible for docking/undocking the robot, suction-irrigation, retraction of tissues, passing sutures into the operative field, and robotic instrument changes. The surgeon is seated at the surgeon console, which provides a superb three-dimensional, 10× magnified, high-definition operative view and allows for the surgeon to have complete control of all camera movements and three additional robotic arms. The surgeon’s thumb and index fingers are inserted into master controls that allow natural hand and wrist movements to be precisely replicated by wristed instruments at the terminal ends of the robotic arms in real time. Highly skilled laparoscopic surgeons may find the robotic technology unnecessary and discover that they are equally as facile with pure laparoscopic suturing and dissection as with the robot (Guillonneau, 2005). Most surgeons, however, feel that the robotic technology significantly facilitates suturing of the vesicourethral anastomosis and aids in other aspects of the surgical dissection such as achieving the critical angles of dissection required to optimize cavernous nerve preservation. For a transperitoneal approach, pneumoperitoneum is established using either a Veress needle inserted at the base of the umbilicus or an open Hasson technique. Following initial trocar placement, CO2 insufflation pressure in general is maintained between 12 and 15 mm Hg. Secondary trocars are then placed under laparoscopic view. For RALP, an example of a trocar configuration is shown in Figure 103–3A. A 12-mm trocar is initially placed at or slightly above the umbilicus for insertion of the stereo endoscope. In a morbidly obese or very tall patient, infraumbilical camera placement may be preferable. Three 8-mm metal robotic trocars are used by the working robotic arms of the surgeon while the assistant provides retraction, suction, and irrigation and passes clips and sutures via the 12-mm and 5-mm trocars placed along the patient’s right side. The surgeon controls camera movement by depressing a foot pedal and using brief, simultaneous arm movements to affect camera positioning and rotation. Endoscopes with either angled (30-degree) or straight-ahead (0-degree) viewing are available and interchangeable at various portions of the procedure. In general, most surgeons use the 0-degree endoscope lens throughout the operation; however, some surgeons prefer to switch to the 30-degree down lens when approaching the bladder neck, NVBs, and apical dissection. Figure 103–3B depicts the trocar configuration for LRP. The surgeon stands at the patient’s left side and operates through the two pararectus trocars while one or two assistants use the lateralmost trocars. The endoscope is held and controlled by an AESOP robotic arm or surgical assistant through the umbilical trocar. For an extraperitoneal approach, a 1.5-cm incision is made at the level of the umbilicus and dissection is carried out down through the anterior rectus sheath. Using blunt finger dissection, a space is created immediately anterior to the posterior rectus sheath and underlying peritoneum. A trocar-mounted balloon dilator device (PDB Balloon, Covidien Autosuture, Mansfield, MA) is inserted into the preperitoneal space anterior to the posterior rectus sheath and advanced down to the pubis along the midline. Using a 0-degree, 10-mm endoscope inserted through the balloon trocar, approximately 500 mL of air is inflated to develop the space of Retzius under laparoscopic view (Fig. 103–4). Secondary trocars are then inserted as described previously under laparoscopic view. The operation then proceeds in the exact manner to that of the transperitoneal anterior approach. In a retrospective comparison between extraperitoneal versus transperitoneal LRP, Hoznek and colleagues (2003) found that the mean operative time was shorter with the extraperitoneal approach (169.6 vs. 224.2 minutes, P < .001) with the greatest time saved during access to the space of Retzius. They suggested that time to full diet was less with the extraperitoneal versus the transperitoneal LRP approach (1.6 vs. 2.6 days, P = .002) because the peritoneum had not been violated and postoperative ileus was minimized. Eden and colleagues (2004) found a statistical significant advantage in operative time, hospital stay, and return of early continence favoring patients undergoing extraperitoneal versus transperitoneal LRP, postulating that earlier return to urinary control may be secondary to less bladder dissection and, perhaps, less bladder dysfunction as compared with transperitoneal LRP. Most studies, however, have found little or no difference in operative time and perioperative outcomes between transperitoneal and extraperitoneal approaches (Cathelineau et al, 2004a; Erdogru et al, 2004; Brown et al, 2005b; Atug et al, 2006). With an extraperitoneal approach, the simultaneous laparoscopic management of concurrent inguinal hernias using prosthetic mesh is feasible (Stolzenburg et al, 2003). Simultaneous inguinal herniorrhaphy has also been reported during transperitoneal LRP (Allaf et al, 2003); however, proper coverage of the mesh prosthesis is necessary using peritoneal flaps, omentum, or a second absorbable mesh to reduce the risk of direct contact between the mesh and bowel with subsequent fistula. The extraperitoneal technique may be preferable in patients with previous extensive abdominal surgery or morbid obesity. With the extraperitoneal approach, the peritoneum acts as a natural barrier, minimizing the potential for bowel injury and preventing the bowels from falling into the operative field and obscuring the surgeon’s view. Furthermore, this approach helps to confine any urine leak that may occur from the vesicourethral anastomosis within the extraperitoneal space. One limitation with the extraperitoneal approach is the reduced working space as compared with the relatively larger working space of the peritoneal cavity gained with transperitoneal access. This is especially relevant when a well-meaning assistant attempts to clear the operative field of blood or smoke. Suctioning can evacuate CO2 gas and rapidly collapse the already limited extraperitoneal working space, thus significantly compromising visualization. A second limitation to the extraperitoneal approach is in patients with a prior history of laparoscopic extraperitoneal mesh herniorrhaphy because the retropubic space is often obliterated, making attempts at extraperitoneal access challenging. Lastly, higher CO2 absorption has been reported with extraperitoneal versus transperitoneal insufflation, requiring a higher minute volume to compensate for hypercarbia and associated acidosis (Meininger et al, 2004). Overall, whether to use an extraperitoneal or transperitoneal approach for LRP or RALP is largely a matter of surgeon preference and experience and there is no consistently demonstrated advantage for either approach. Following abdominal access and trocar placement for the transperitoneal anterior approach, the pelvic contents are inspected (Fig. 103–5) and adhesions are lysed if present. The initial step is entry and development of the space of Retzius. The bladder is dissected from the anterior abdominal wall by dividing the urachus high above the bladder and incising the peritoneum bilaterally immediately lateral to the medial umbilical ligaments using monopolar electrocautery. The presence of prevesical fatty alveolar tissue confirms the proper plane of dissection. Applying posterior and cephalad traction on the urachus, the retropubic space is rapidly developed with a combination of blunt and sharp dissection along the relatively avascular plane within the space of Retzius (Fig. 103–6). Lateral dissection of the bladder is carried out down toward the crossing of the median umbilical ligament and vas deferens in order to ensure optimal mobility of the bladder and to minimize future tension at the vesicourethral anastomosis. The fat overlying the prostate is removed using sharp dissection and electrocautery as needed, and the superficial branches of the DVC coagulated using bipolar electrocautery. Figure 103–5 Initial transperitoneal view detailing the relevant landmarks within the male pelvis. NVB, neurovascular bundle. At this point, visible landmarks include the anterior aspect of the bladder and prostate, puboprostatic ligaments, endopelvic fascia, and pubis (Fig. 103–7). The endopelvic fascia and puboprostatic ligaments are sharply divided, exposing levator muscle fibers attached to the lateral and apical portions of the prostate. These fibers are meticulously and bluntly dissected from the surface of the prostate exposing the deep DVC and urethra at their confluence with the apex of the prostate. Electrocautery is avoided if possible to minimize thermal damage to the external sphincter and nearby NVBs. Accessory pudendal arteries traveling longitudinally along the anteromedial aspect of the prostate are easily recognized during LRP and RALP. Attempt at preservation of these arteries is important for erectile function because in some men these arteries may be the dominant source of arterial blood supply to the corpora cavernosa (Nehra et al, 2008). These accessory arteries can usually be preserved, although separation of the artery from the prostatic apex can be somewhat challenging. Most commonly, though, careful and meticulous dissection allows enough mobilization of the artery away from the deep DVC and prostatic apex that both good anatomic dissection of the prostatic apex and preservation of the accessory vessel are feasible. As with open surgery, different methods have been described for control of the DVC. A common observation, though, is that the profuse bleeding, which is sometimes encountered during open surgery, is not usually problematic because of the tamponade effect on venous bleeding offered by the pneumoperitoneum. In fact, some techniques involve simple cutting of the DVC with or without the use of electrocautery and placement of a suture only if necessary. Typically, there are one or two small arteries in the DVC. Most described techniques use placement of an absorbable suture for hemostasis before initiation of the surgical dissection of the prostate. During RALP, the ProGrasp forceps can be used for fixed cephalad retraction of the prostate and bladder to achieve optimal exposure of the DVC and prostatic apex before DVC ligation. Similar retraction can be applied by the surgical assistant during LRP. The deep DVC is suture ligated using a 0-polydioxanone suture or polyglactin suture as close to the pubis and as far from the prostatic apex as possible (Fig. 103–8). Securing the DVC as far away from the prostatic apex as possible can help minimize iatrogenic entry into the prostatic apex during later division of the DVC. The needle is passed beneath the DVC and anterior to the urethra. An alternative method to DVC ligation is the use a laparoscopic linear stapling device, which ligates and divides the DVC all in one step (Ahlering et al, 2004b; Nguyen et al, 2008). Regardless of the method used, it is important to avoid damage to the anterior urethral sphincter muscle from placing the sutures or staples too deep. With most techniques, the DVC is not divided until later in the operation and immediately before prostatic apical dissection and division of the urethra. A back-bleeding suture may be placed along the anterior base of the prostate to help identify the contour of the prostate and to aid in subsequent bladder neck identification and transection. The posterior bladder neck is inspected for the presence of a median lobe and to locate the ureteral orifices. If a vertical drop-off of the posterior bladder neck mucosa is noted, this would suggest the absence of a median lobe. Alternatively, if a mass effect from a large median lobe is identified, further exposure may be required to visualize beneath the protruding median lobe and identify the posterior bladder neck. The median lobe is lifted anteriorly using either the ProGrasp forceps or the surgical assistant. The posterior bladder neck is horizontally divided with monopolar electrocautery, staying along the midline to avoid bleeding from the lateral pedicles (Fig. 103–9). Dissection is carried out in a 45-degree downward angle to avoid entry into the base of the prostate, as well as creating a buttonhole in the posterior wall of the bladder. In case of a prior TURP, the bladder neck margin is less evident and often distorted as a result of prior resection and scarring. Careful inspection is made of the posterior bladder neck paying specific attention to the location of the ureteral orifices because they are often found close to the posterior bladder neck margin. Attempt at bladder neck sparing should be avoided in post-TURP and median lobe cases. When in doubt, the posterior bladder neck should be divided slightly more proximally in these particular cases so as to avoid inadvertent entry into the prostate gland. Following bladder neck transection, the seminal vesicles and vasa deferentia are individually identified, dissected, and divided, avoiding electrocautery if possible to prevent damage to the nearby NVB (Fig. 103–10). One unique distinction between the transperitoneal anterior and retrovesical approach is in dissection of the seminal vesicles and vasa deferentia. During a transperitoneal retrovesical approach, the initial step of the operation is complete dissection of the vasa deferentia and seminal vesicles deep within the cul-de-sac. Following abdominal access, the vasa are dissected from lateral to medial toward their confluence at the ejaculatory ducts. The seminal vesicles are found immediately lateral to the vasa and are dissected free from the nearby NVB using hemoclips while avoiding the use of thermal energy (Fig. 103–11). With the dissection of the seminal vesicles and vasa now completed, once the bladder neck is divided from the prostate, these structures are simply grasped and brought through the opening. This retrovesical approach to the seminal vesicles and vasa deferentia is particularly useful in cases of a median lobe where identification and dissection of these structures by the anterior approach may be more challenging due to the protruding median lobe. Separation of the posterior prostate from the anterior rectal wall is a key surgical maneuver to avoid rectal injury but also to permit adequate identification of the prostatic pedicles and establish the medial border of the NVB. Development of this plane in an antegrade fashion is a maneuver often unfamiliar to surgeons experienced with open surgery but one that is rapidly adaptable to laparoscopic and robotic approaches. Anterior retraction of the vasa deferentia and seminal vesicles by either a surgical assistant or the robotic ProGrasp forceps helps with identification of the proper plane for the initial dissection (Fig. 103–12).
Evolution of Minimally Invasive Laparoscopic Prostatectomy
Patient Selection
Indications and Contraindications
Instrumentation
Laparoscopic Radical Prostatectomy
Bipolar forceps
Robotic-Assisted Laparoscopic Prostatectomy
Preoperative Preparation
Operating Room Personnel
Anesthesia Considerations
Surgical Technique
Robotic-Assisted versus Pure Laparoscopic Approach
Transperitoneal Approach
Abdominal Access, Insufflation, and Trocar Placement
Extraperitoneal Approach
Pros and Cons of Extraperitoneal versus Transperitoneal Approach
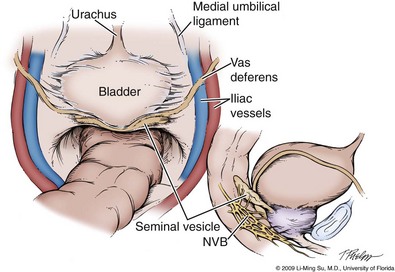
Developing the Space of Retzius
Ligation of the Deep Dorsal Venous Complex
Bladder Neck Identification and Transection
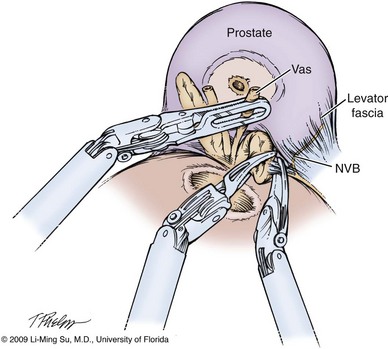
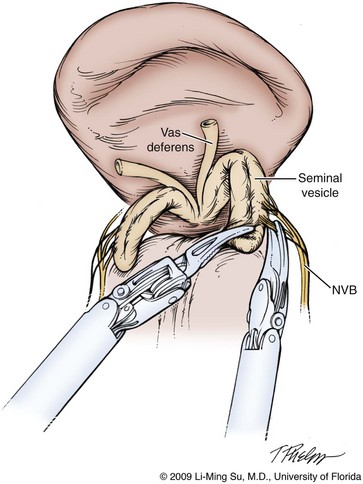
Dissection of the Seminal Vesicles and Vasa Deferentia
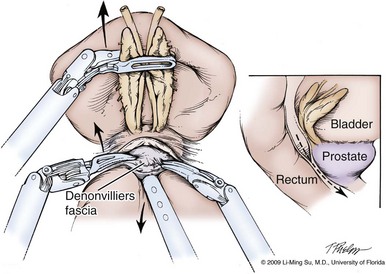
Development of the Plane between the Prostate and Rectum