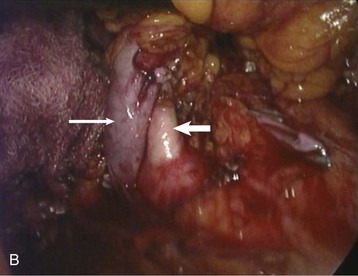
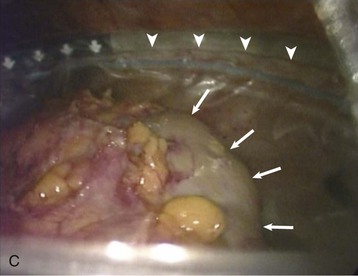
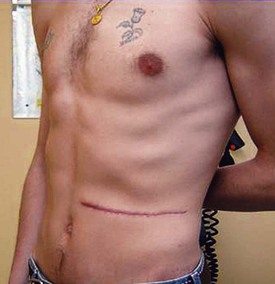
Adam D. Barlow, Michael L. Nicholson The usual and most frequent source of kidneys for transplantation has been donation after brain death (DBD), formerly known as donation from a heart-beating cadaveric donor. The increasing worldwide discrepancy between the availability of and the need for renal allografts1 has led to the increasing use of alternative sources of organs, including donation after cardiac death (DCD) donors (previously known as non–heart-beating donors) and living donors. The evaluation and selection of donors are discussed in Chapter 102. Here we discuss surgical aspects of retrieval and transplantation of kidneys. The potential DBD donor is maintained by artificial ventilation in a critical care setting until death has been diagnosed by brainstem death criteria,2 the consent of the next of kin for donation has been given, and the necessary legal and institutional approvals have been obtained. In the operating room the initial step is cannulation of the aorta and inferior vena cava while the heart is still beating. This allows perfusion of the organs with cold preservative solution immediately before cardiac arrest, minimizing warm ischemia. The priorities of the organ retrieval team are influenced by the range of organs being donated. Heart, lung, and liver retrieval take priority over kidney retrieval, which may significantly lengthen the ischemic time. The kidneys are removed en bloc, and typically the artery is retrieved with a cuff of aorta (Carrel patch), with the maximum achievable length of renal vein, and 10 to 15 cm of ureter. The length of the right renal vein may be maximized by including a portion of inferior vena cava in continuity. Care is taken to avoid damage to polar and other accessory arteries, especially the lower pole artery, which may supply the ureter; stripping of adventitial tissue from the ureter must also be avoided, because this may also compromise its blood supply. The kidneys are flushed with ice cold preservation fluid until the effluent is clear and then are stored for transport in crushed ice or on a perfusion machine (see the discussion of renal preservation). Before consensus was reached regarding the definition of brainstem death, DCD donors were the main source of transplant organs. These donors were intensive care unit based and had suffered head injuries or cerebrovascular accidents deemed irrecoverable, but organ retrieval could proceed only after cardiorespiratory death. This changed with the introduction of brainstem death legislation, but the use of DCD kidneys has recently increased again in response to the shortage of suitable organs for transplantation. An international consensus has defined categories of DCD donors3 to facilitate legal and ethical discussion and to highlight possible differences in organ viability (see Box 102-3). DCD kidneys sustain a period of warm ischemia, the period between cardiac arrest and the time that in situ cold perfusion is started. The duration of ischemia correlates with rates of primary nonfunction, delayed graft function, acute rejection, allograft, and patient survival. The main requirement of organ procurement from DCD donors is therefore to achieve rapid in situ perfusion of the kidneys to limit warm ischemia. This requires an emergency response team of surgeons and transplant coordinators, with considerable on-call and logistic commitments. Donation after cardiac death donors may be either uncontrolled (Maastricht categories I and II) or controlled (Maastricht categories III through V). In controlled donors, cardiac arrest is expected, and it is therefore possible to reduce the warm ischemia time to only a few minutes because the surgical retrieval team will be on standby. Unexpected donor cardiac arrest may result in prolonged warm ischemia times. The duration of reversible warm ischemia time that the human kidney can sustain is unknown, but DCD kidneys with warm ischemia exceeding 60 minutes are considered by many to be of marginal suitability. Centers involved with DCD donation should adhere to the Maastricht protocol,4 which includes the following principles: After a period of unsuccessful resuscitation, confirmation of cardiac death, and observation of the 10-minute rule, cardiac massage and ventilation with 100% oxygen are recommenced in an attempt to deliver oxygenated blood to the kidneys. A mechanical resuscitation device may be used. In situ renal cooling is effected by placing a double-balloon, triple-lumen perfusion catheter into the aorta via a femoral artery cut-down (Fig. 103-1), with instillation of preservation solution. Alternatively, the donor can be moved to an operating room as soon as death has occurred, and the aortic perfusion catheter is placed directly at laparotomy rather than via a femoral artery cut-down. For controlled DCD donors, the transplantation team awaits cardiac arrest; after confirmation of death, the 10-minute rule is observed, and then the perfusion catheter is inserted via the femoral artery. Alternatively, the patient can be taken to the operating room before cardiac death if the next of kin gives consent. In the United States in 2011, 32% of renal transplants were from living donors,5 compared with 40% in the United Kingdom.6 After a rapid increase in living donor transplantation at the beginning of this decade, rates have become more static. Superior recipient post-transplantation outcome compared with use of kidneys from deceased donors,7 the potential for preemptive transplantation before dialysis, and the ability to plan the procedure (allowing optimization of recipient condition) are major advantages and justify continued efforts to expand use of living donors. The medical evaluation of the living donor is discussed in Chapter 102 (see Boxes 102-5 and 102-6). Preoperative imaging of living donors confirms the presence of two functioning kidneys, indicates pathology that would preclude donation, and provides anatomic information necessary for planning the procedure. Imaging assumes paramount importance before minimal access donor nephrectomy because of the reduced operative exposure and particular difficulties in the identification of complex renal vein tributaries. The location, size, and number of renal veins and tributaries needs to be accurately described preoperatively. Angiography combined with excretion urography is now obsolete. For preoperative description of the main renal artery and vein anatomy, magnetic resonance angiography and computed tomographic angiography are comparable,8 but computed tomographic angiography is more sensitive and specific for complex vascular anatomy and provides excellent correlation between imaging and surgical findings9 (Fig. 103-2). Living donor nephrectomy has traditionally been performed through an open incision, necessitating a prolonged period of recovery. This and the cosmetic implications of a large flank wound may discourage potential donors (Fig. 103-3). To reduce such disincentives, there has been a move toward minimally invasive donor nephrectomy, first performed as a transperitoneal laparoscopic procedure (laparoscopic donor nephrectomy [LapDN]).10 LapDN is associated with decreased severity and duration of postoperative pain, shorter in-patient stay, quicker return to work and normal activities, and improved cosmetic result when compared with open donor nephrectomy11 (Box 103-1). Furthermore, the overall societal cost of LapDN is lower,12 and recipient quality-of-life scores are higher.13 The procedure is, however, technically demanding, and there is potential for damage to the renal parenchyma, vessels, and ureter during dissection. It takes longer than open nephrectomy and exposes the allograft to a longer period of warm ischemia.11 Nevertheless, retrospective data suggest that minimal access donor nephrectomy not only offers postoperative advantages to the donor, but also increases the number of transplants performed by reducing donor disincentives; estimates range from a 25% to a 100%14 increase in transplantation activity. The widespread introduction of LapDN at the beginning of this decade saw an initial dramatic increase in the number of live kidney donors. However, rates have been static in both the United States and the United Kingdom over the last 5 years, suggesting that we may have seen the maximum benefits of this effect. Three minimal access approaches have been described: transperitoneal, extraperitoneal, and hand-assisted living donor nephrectomy. Pneumoperitoneum is established and four laparoscopic ports are usually required (Fig. 103-4). After laparoscopic dissection a Pfannenstiel incision is made through which the kidney is brought out within an endoscopy retrieval bag after control and division of the artery vein and ureter. The hand-assisted technique allows tactile sense to facilitate dissection, retraction, and exposure. It is said to be easier to learn and can be safely and efficiently performed by surgeons with less laparoscopic experience. The hand-assist device allows the operator’s nondominant hand to enter the abdomen through an airtight system. The retroperitoneal approach avoids breaching the peritoneum, displays the renal anatomy in a very different manner, and may be easier for retrieving the full length of the vessels, especially on the right side. The disadvantage is that a more limited operating space is available than with the transperitoneal or hand-assisted laparoscopic techniques. There are no absolute contraindications other than those applying to the open operation. The relative contraindications are dictated by donor factors and the experience of the surgeon. The donor must be fit for anesthesia, including the physiologic stress of pneumoperitoneum. Obesity is a relative contraindication for both open and laparoscopic surgery, and the hand-assisted approach may be better suited in such patients. Previous abdominal surgery is a further relative contraindication because of the potential for adhesions. Multiplicity of renal vessels should not hinder LapDN. Transient intraoperative oliguria secondary to decreased renal blood flow is a frequent occurrence during laparoscopic procedures. Proposed mechanisms include decreased cardiac output, renal vein compression, ureteral obstruction, renal parenchymal compression, and systemic hormonal effects. Intracranial pressure increases during pneumoperitoneum, with release of vasoconstrictor agents that decrease renal blood flow. Use of a lower pressure reduces the adverse effects of pneumoperitoneum on renal perfusion. In donor nephrectomy, impaired renal blood flow may compromise early allograft function and compound the damaging effects of warm and cold ischemia and operative manipulation of the kidney. Laparoscopically derived donor kidneys have higher serum creatinine up to 1 month post-transplantation compared with open surgery, but thereafter graft function is equivalent.15 The pioneers of LapDN used high volumes of crystalloid preoperatively and intraoperatively to maintain renal perfusion in the presence of pneumoperitoneum. The authors have seen two episodes of unilateral pulmonary edema in the dependent lung, and we now recommend volume loading the donor with 2 liters of crystalloid the night before surgery and only using replacement fluid during surgery. This protocol has led to no apparent detriment to graft function. There is no consistent evidence that graft function differs among kidneys retrieved by open, laparoscopic, or hand-assisted donor nephrectomy. The exception is that rates of delayed graft function and acute rejection may be higher in pediatric recipients, especially the 0- to 5-year age group. Pretransplantation ischemia could, in theory, render the donor kidney more immunogenic by inducing major histocompatibility complex (MHC) class II expression. However, despite the longer warm ischemia time, acute rejection rates and severity of rejection are not higher in laparoscopic than in openly retrieved living donor kidneys. Ureteral ischemia was more common in early experience of LapDN but can be avoided if care is taken to ensure that sufficient periureteral tissue is taken and that the dissection does not occur too close to the renal pelvis. Multiple arteries need not be a barrier to successful use of grafts from laparoscopic donors. In open donor nephrectomy, the right kidney is retrieved in 20% to 30% of procedures, whereas LapDN uses the right kidney in less than 10%,16 reflecting concern over the operative safety of the right-sided laparoscopic operation, principally the difficulties involved in obtaining an adequate length of renal vein. It has been argued that this practice has led to compromise of the principle that the better kidney should remain with the donor. After uneventful open nephrectomy, the donor can expect to be discharged from the hospital in 5 to 6 days and is able to return to work after 3 to 6 weeks, although return to work has been shown to be 2 to 3 weeks later in open nephrectomy compared with LapDN.11 After LapDN the donor usually leaves hospital in 2 to 4 days and can return to work in 3 to 6 weeks. The choice of operative procedure depends on the local expertise of the surgeons. There is accumulating evidence that the laparoscopic operation removes some of the disincentives to donation, and this approach is likely to be adopted widely in the future. Preservation of deceased donor organs is crucial to allow time for matching, sharing of organs, and preparation of the recipient. Damage from hypothermia and reperfusion must be minimized. There is little standardization of the type of preservation solution used. Marshall’s hyperosmolar citrate solution and histidine-tryptophan-ketoglutarate are popular choices in Europe, but University of Wisconsin (UW) solution is more commonly used in the United States because extended preservation times are more often required. Organs can be preserved by cold storage (kept in crushed ice after flushing with preservation solution) or by machine-driven pulsatile perfusion. The proposed benefits of machine perfusion come from allowing aerobic function through provision of oxygen and substrate and removal of metabolic end products. Although machine perfusion has been used for many years, there is still no consensus about its superiority to cold storage nor about the best perfusion parameters. Recent randomized trials have yielded conflicting results; a U.K. trial of DCD kidneys showed no benefit,17 whereas a European trial demonstrated reduced delayed graft function in deceased donor transplants after machine perfusion.18 A novel approach to kidney preservation, only recently introduced into clinical practice, is ex vivo normothermic perfusion. Early results are promising,19 but the technique needs to be studied in larger clinical trials. Decisions on the use of a kidney from a marginal donor can be supported by data from machine or normothermic perfusion; high perfusion pressures are associated with primary nonfunction and delayed graft function.
Kidney Transplantation Surgery
Sources of Kidneys for Transplantation
Donation Before Cardiac Death Donors
Donation After Cardiac Death Donors
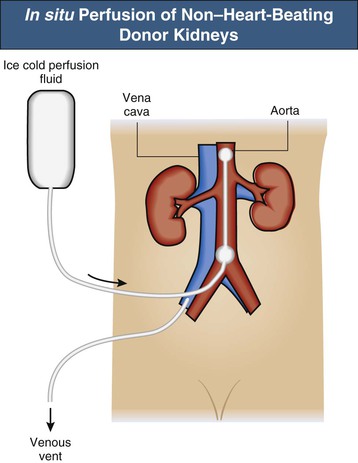
Donation After Cardiac Death Protocol
Uncontrolled DCD Donors
Controlled DCD Donors
Living Kidney Donors
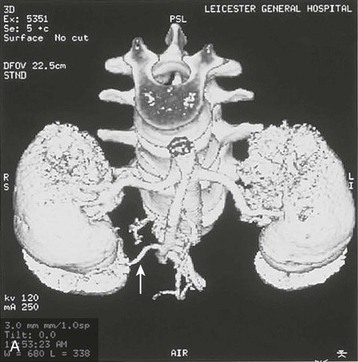
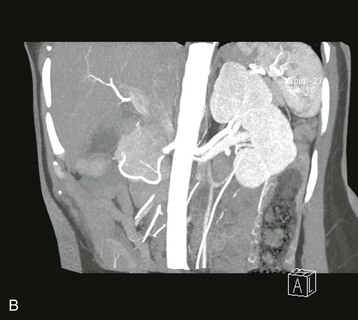
Preoperative Imaging
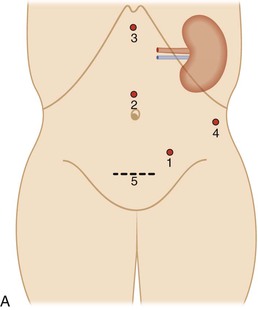
Minimal Access (Laparoscopic) Donor Nephrectomy
Transperitoneal Laparoscopic Donor Nephrectomy
Hand-Assisted Laparoscopic Donor Nephrectomy
Retroperitoneoscopic Operative Technique
Contraindications to Minimal Access Donor Nephrectomy
Effect of Pneumoperitoneum
Graft Function and Acute Rejection
Technical Issues
Postoperative Recovery
Choice of Donor Operative Technique
Renal Preservation
Renal Transplantation Procedure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Kidney Transplantation Surgery
Chapter 103