Colm C. Magee
Kidney Disease in Liver, Cardiac, Lung, and Hematopoietic Cell Transplantation
It is now well recognized that kidney disease can complicate all forms of solid organ nonrenal transplantation.1–3 Kidney disease can occur in the immediate pretransplantation period, in the early postoperative period, or during the long term. In general, it is associated with longer hospitalization, higher morbidity, higher mortality, and greater expense. Although there are organ-specific factors (such as the high prevalence of hepatitis C virus infection in liver transplant recipients) that have an impact on the incidence and severity of kidney disease, some useful generalizations can be made.
Generic Issues of Kidney Disease in Nonrenal Solid Organ Transplantation
Use of Serum Creatinine and Derived Equations to Measure Glomerular Filtration Rate
Transplant candidates and recipients often have low muscle mass and low creatinine generation. Hence, a mildly elevated serum creatinine concentration may hide severe kidney disease. This is particularly relevant in the pretransplant patient with liver failure.3,4 Such patients should have glomerular filtration rate (GFR) estimated by creatinine clearance (which will tend to overestimate) or preferably by iothalamate clearance (where it is available). Of the various serum creatinine–based equations to estimate GFR, the Modification of Diet in Renal Disease (MDRD) equation appears to correlate best with true post-transplantation GFR; however, even this equation has limited accuracy in all forms of solid organ transplantation.4
Nephrotoxicity of Calcineurin Inhibitors
The introduction of the calcineurin inhibitor (CNI) cyclosporine in the 1980s revolutionized the field of organ transplantation, and the CNIs cyclosporine and tacrolimus remain a cornerstone of immunosuppression in recipients of nonrenal allografts.1 However, there is little doubt that CNIs contribute significantly to both acute kidney injury (AKI) and chronic kidney disease (CKD) in these recipients. There is speculation that CNI nephrotoxicity is more severe in nonrenal than in renal transplantation because (1) dosage and blood concentrations of CNIs tend to be higher in nonrenal transplantation and (2) the lack of normal innervation of the transplanted kidney may protect it from early CNI injury. CNIs also exacerbate post-transplantation hypertension and diabetes mellitus, which cause renal damage in the long term.
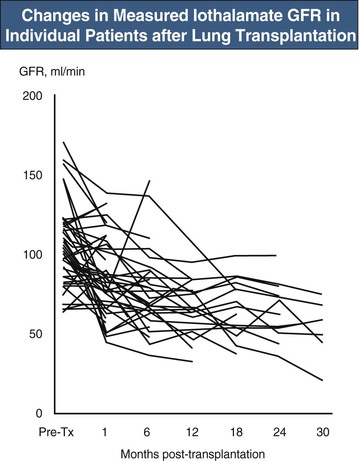
Acute CNI nephrotoxicity is thought to be mainly a pre-renal syndrome caused by vasoconstriction of the afferent glomerular arteriole (see also Chapter 101). Tubular damage and microvascular disease may occur in more severe cases. In the early post-transplantation period, AKI is usually multifactorial, and it is difficult to quantify the degree to which CNI toxicity is contributing to the problem. In practice, doses of CNIs are often temporarily reduced in the setting of AKI. Acute CNI-induced thrombotic microangiopathy (TMA) is rare in nonrenal transplant recipients. Chronic CNI nephrotoxicity is probably a result of prolonged renal ischemia and other effects, such as direct stimulation of renal fibrogenesis. Typically the patient is hypertensive (CNIs worsen hypertension) and there is a steady fall in GFR, most marked in the first 6 to 12 months after transplantation (Fig. 111-1).5 Dipstick urinalysis shows minimal or no hematuria and minimal or mild proteinuria. Urine protein-creatinine ratio or 24-hour urine collections confirm low-grade proteinuria. Renal histology shows interstitial fibrosis, arteriolar hyalinosis, arteriosclerosis, and secondary focal glomerulosclerosis.6 There may also be features of chronic TMA.6 In practice, renal biopsies are rarely performed unless there are clinical features suggestive of a renal disorder other than CNI toxicity.
It is not surprising that the presumed high prevalence of chronic CNI nephrotoxicity has generated interest in low-dose or zero-dose CNI protocols. Just as in renal transplantation, these typically involve use of mycophenolate mofetil (MMF) or mammalian target of rapamycin (mTOR) inhibitors.1,3,7 However, there is still understandable reluctance to pursue such protocols because CNIs are considered such effective immunosuppressive agents, and organ replacement therapy analogous to dialysis is not available for other organ transplant recipients if severe rejection occurs. MMF has the advantage of being non-nephrotoxic. mTOR inhibitors have several disadvantages; they impair wound healing, potentiate the nephrotoxicity of cyclosporine, and sometimes induce proteinuria. Although some studies suggest that everolimus is better tolerated than sirolimus, similar nephrotoxic interactions have been reported between both sirolimus and everolimus with cyclosporine.8 CNI-sparing strategies are discussed in more detail later.
Another strategy is to use tacrolimus as the de novo CNI or to switch from cyclosporine to tacrolimus if there is evidence of nephrotoxicity (cyclosporine is still commonly prescribed as the de novo CNI in heart and lung transplantation). The rationale here is that tacrolimus provides equivalent or even better immunosuppression at doses and concentrations that are less nephrotoxic than cyclosporine. Furthermore, tacrolimus is associated with less hypertension and hyperlipidemia (which might exacerbate CKD) than cyclosporine. Conversely, it is associated with more post-transplantation diabetes mellitus. Studies in nonrenal transplant recipients have yielded conflicting results as to which is more nephrotoxic.
The usefulness of calcium channel blockers in ameliorating CNI nephrotoxicity in renal transplantation remains controversial, and there is limited evidence of such benefit in nonrenal transplantation. Experimental studies suggest that agents that block the renin-angiotensin system (RAS) may provide protection against the arteriolar hyalinosis and tubulointerstitial injury associated with chronic CNI use. Control of hypertension per se is probably more important than use of any particular antihypertensive agent.
Acute Kidney Injury in the Immediate Pretransplantation Period
It is not surprising that AKI can occur in the days to weeks immediately before liver, heart, or (less commonly) lung transplantation. Typically, the cause of AKI is either pre-renal (e.g., renal hypoperfusion or hepatorenal syndrome) or intrarenal (ischemic or toxic acute tubular necrosis) or a combination of both. AKI may develop on a background of CKD. A mildly elevated serum creatinine may mask a significant fall in GFR in malnourished patients with severe organ failure.3 Reported rates of AKI vary widely; rates may be modified by varying definitions of AKI, organ-specific risks of AKI, and center variability in the type of patients listed for transplantation.
Management of AKI focuses on treatment of the underlying cause and provision of dialytic support according to standard criteria. Because the patients are critically ill, continuous renal replacement therapy (CRRT) may be preferred to intermittent hemodialysis, but there are no randomized controlled trials showing improved outcomes with this modality in this setting. When severe or prolonged AKI occurs before planned transplantation, estimation of the degree of reversibility becomes very important, because presumed irreversible severe kidney injury is generally a contraindication to transplantation or shifts the management toward simultaneous dual-organ (e.g., liver and kidney) transplantation. This is an area of ongoing debate in liver and, to a lesser extent, cardiac transplantation and is discussed in detail later. The general advantages and disadvantages of simultaneous dual-organ transplantation are shown in Table 111-1.
Table 111-1
Advantages and disadvantages of simultaneous renal and nonrenal transplantation.
AKI, Acute kidney injury; ESRD, end-stage renal disease.
| Advantages and Disadvantages of Simultaneous Kidney and Other Solid Organ Transplantation | |
| Advantages | Disadvantages |
| Potentially provides much better renal function over the short and long terms | Surgery more technically complex and prolonged Not needed when the AKI is reversible |
| Single donor—potential for lower cumulative dose of immunosuppression (as opposed to kidney transplantation later) | Deprives patients with “definite” ESRD of a kidney transplant |
Although kidney biopsy might be useful in determining the degree of reversibility, in practice it is not commonly performed. This reflects the perceived technical difficulties of performing the procedure in critically ill—often coagulopathic—patients, in whom a biopsy-related bleed could have catastrophic consequences.
Acute Kidney Injury in the Early Post-transplantation Period
Acute kidney injury is also common in the days to weeks after transplantation. This reflects several factors: pretransplant renal function may have been compromised, the transplanted organ may have poor initial function, high doses of CNIs and other nephrotoxic agents may be prescribed, and the invasive surgery and high-dose immunosuppression predispose to severe infection (Table 111-2). AKI is associated with higher mortality in the early postoperative period.9–11 This is likely to reflect both the association of AKI with other severe complications and also its direct effects on mortality. In those who survive, AKI is associated with an increased risk of developing CKD.12
Prevention or minimization of post-transplant AKI involves careful selection of patients for transplantation, meticulous perioperative care, and avoidance of nephrotoxic agents. Management of established AKI focuses on treatment of the underlying cause and provision of dialytic support according to standard criteria. Intermittent hemodialysis and CRRT are both used. Delayed introduction or reduced doses of CNIs are sometimes used, usually under the cover of induction antibody immunosuppression. How much this strategy improves renal function remains unclear.
Chronic Kidney Disease
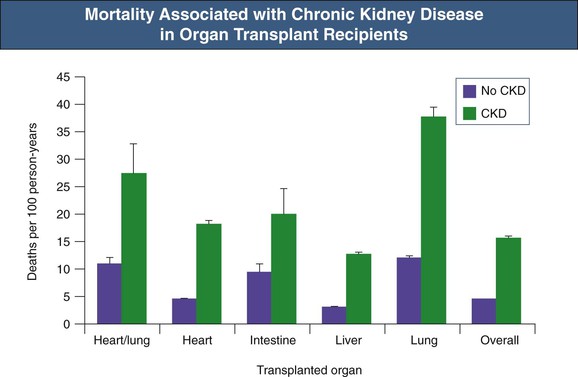
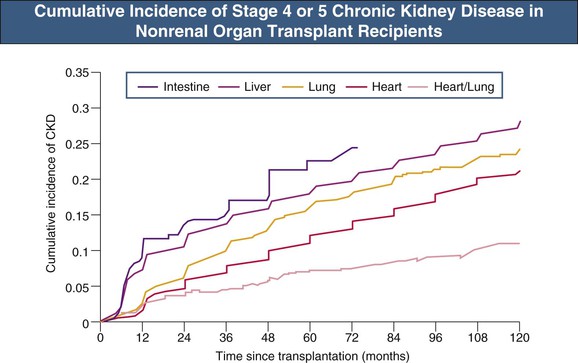
With the increasing number of solid organ transplants being performed worldwide and the longer survival of recipients, the absolute number of recipients with CKD has increased. In the largest and most comprehensive study to date, the cumulative incidence of post-transplant CKD (defined as estimated glomerular filtration rate [eGFR] <30 ml/min/1.73 m2) at 5 years varied from 7% to 21% (Fig. 111-2).12 By multivariate analysis, the following pretransplant variables increased the risk of development of CKD: increasing age, female gender, white or black (as opposed to Asian) ethnicity, lower GFR, diabetes mellitus, hypertension, hepatitis C virus infection, and need for dialysis. The following post-transplant variables were also implicated: postoperative AKI and initial use of cyclosporine (as opposed to tacrolimus).12 These risk factors have been confirmed in other studies.
Many studies have also confirmed that whatever the transplanted organ, development of CKD, and especially of end-stage renal disease (ESRD), portends a poor prognosis (Fig. 111-3). Recipients with CKD have a relative risk of death of 4.6 compared with those without CKD.12 The risk is highest in those with ESRD, but even in those not on dialysis, the relative risk of death was doubled.12
Although it has not been well studied, CKD in this setting is presumably associated with the same complications as in nontransplant recipients, including anemia, hypertension, fluid overload, and bone and mineral disorders. Indeed, these complications might be more severe in nonrenal transplant recipients because of other risk factors. For example, antiproliferative immunosuppressants exacerbate anemia and corticosteroids exacerbate metabolic bone disease.
Management of Chronic Kidney Disease
The MDRD equation (despite its limitations) is useful in identifying CKD in these patients. In all patients with rising serum creatinine or falling GFR, referral to a nephrologist should be considered. The initial evaluation should include thorough review of renal function in the peritransplantation period and exposure to nephrotoxins. If urinalysis shows moderate or severe proteinuria or hematuria or both, renal biopsy should be considered; it can be highly informative in identifying renal diseases other than CNI nephrotoxicity.13
Close consultation with the primary transplant team is important. When CNI toxicity is deemed the main cause of CKD (as it is in the majority of patients), reduction in CNI dosage for patients at low immunologic risk should be considered. To maintain adequate immunosuppression, if the patient is receiving azathioprine, a change to MMF should be considered; if the patient is already receiving MMF, the dose should probably be increased. An alternative strategy (one that I favor less) is to substitute an mTOR inhibitor for the CNI; this should not be done in the early post-transplant period because of the adverse effects of these drugs on wound healing. Finally, some have advocated switching of cyclosporine to low-dose tacrolimus, although the supporting data are less compelling. It should be noted that the majority of studies of CNI reduction or cessation in these settings are small, have limited follow-up, and are of limited quality.14
The general management of CKD has not been well studied in nonrenal transplant recipients, but it seems reasonable to use the same strategies used in the nontransplant CKD population. Thus, hypertension, anemia, and hyperparathyroidism should be managed according to standard guidelines. Although there is experimental evidence that angiotensin-converting enzyme (ACE) inhibitors might have important antifibrotic effects in nonrenal transplant CKD, there are no trials showing improved renal outcomes with ACE inhibitors or angiotensin receptor blockers compared with other antihypertensive agents. In practice, lifestyle modification and several antihypertensive drugs are usually required for achievement of target blood pressure.
In those with severe CKD, planning for renal replacement therapy including renal transplantation (where appropriate) should begin early. The majority are treated with hemodialysis rather than with peritoneal dialysis. With either modality, mortality is much higher compared with matched nontransplant controls.15 Nevertheless, some have reported reasonable outcomes with home therapies (both hemodialysis and peritoneal dialysis).16 Pending further studies, it seems that the choice of dialysis modality should be dictated by patient-specific factors.
There seems little doubt that in those fit for the procedure, sequential kidney transplantation is the best form of renal replacement therapy. Because patients have already undergone transplantation, the addition of the renal allograft does not imply a huge increase in immunosuppression (typically, this is increased only in the perioperative period). When it is feasible, a preemptive living donor transplant is probably the best option. Initial mortality is higher in nonrenal transplant recipients receiving a kidney transplant as opposed to staying on the waiting list (reflecting the complications of surgery and more immunosuppression), but mortality in the medium and long term is much lower.12 The number of patients with a nonrenal transplant being wait-listed in the United States has increased significantly over the last 15 years.15
Prevention of Chronic Kidney Disease
The rate of development and progression of CKD can probably be reduced by minimizing post-transplantation AKI (see earlier) and by rigorous control of hypertension in the long term. Clearly, safe and effective non-CNI or low-dose CNI protocols would be an important advance in preventing severe CKD. Studies using induction antibody with corticosteroids, MMF, and low-dose tacrolimus are encouraging in this regard.3,17
BK Virus Nephropathy
BK virus nephropathy is a much-feared complication of kidney transplantation. Fortunately, although BK viruria is occasionally noted after liver, heart, or lung transplantation, the absolute risk of BK viremia and BK virus nephropathy remains low. This probably reflects the lack of a “second hit” to the native kidneys, such as human leukocyte antigen (HLA) mismatching or inflammation. Nevertheless, BK virus nephropathy should be considered in the differential diagnosis of “unexplained” renal dysfunction after solid organ transplantation, especially because more intensive maintenance immunosuppressive protocols involving combined MMF and tacrolimus are being increasingly used.
Acute Kidney Injury in the Late Post-transplantation Period
Severe AKI occasionally occurs late after transplantation. The major causes are shown in Table 111-2. Severe rhabdomyolysis has been reported, usually when statins are prescribed with cyclosporine and an inhibitor of the cytochrome P-450 system, such as diltiazem or ketoconazole and other azole-based antifungal agents. Treatment of late AKI is of the underlying cause plus the usual supportive measures.
Kidney Disease in Liver Transplantation
Acute kidney injury is common before liver transplantation. Indeed, use of the MELD (Model for End-Stage Liver Disease) scoring system (which measures severity of hepatic dysfunction and risk of death) to guide allocation of deceased donor liver allografts means that a raised serum creatinine concentration increases the chances of the patient being offered a liver transplant.18 The most common causes of AKI before transplantation are hepatorenal syndrome (see Chapter 76) and acute tubular necrosis or both. Prolonged hepatorenal syndrome may cause acute tubular necrosis. Glomerulonephritis (GN) is relatively common (cirrhosis is associated with IgA nephropathy, and hepatitis C with membranoproliferative glomerulonephritis [MPGN]) but is rarely the cause of severe pretransplantation kidney disease. If renal replacement therapy is required, CRRT is often preferred, in part because it is thought to have less effect than intermittent dialysis on intracranial pressure.
Post-transplantation AKI is also common and is usually multifactorial.9 In one series, 37% of liver transplants were complicated by some degree of AKI, 19% necessitating renal replacement therapy.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree