David C. Wymer
Imaging
Imaging evaluation of patients with renal disease has changed significantly in recent years. Intravenous urography is infrequently used and has mostly been replaced by ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine scanning. Rapidly changing computer-based data manipulation has resulted in major technologic advances in each of these modalities. Three-dimensional or even four-dimensional (time-sensitive) image analysis is now available. Molecular imaging, which visualizes cellular function using biomarkers, is providing functional as well as anatomic information.
The American College of Radiology (ACR) has published Appropriateness Criteria,1 guidelines that suggest the choice of imaging to provide a rapid answer to the clinical question while minimizing cost and potential adverse effects to the patient, such as contrast-induced nephrotoxicity and radiation exposure. Tables 5-1, 5-2, and 5-3 list relative radiation exposures, first-choice imaging modalities in renal disease, and risk estimates, respectively. Risks of imaging and cost need to be balanced against benefits.
Table 5-1
Relative radiation doses of imaging examinations.
PA, Posteroanterior; mSv, millisieverts; KUB, kidney, ureter, bladder (plain film); CT, computed tomography; PET, positron emission tomography; MRI, magnetic resonance imaging.
| Relative Radiation Doses of Imaging Examinations | |
| Examination | Effective Dose (mSv) |
| Chest: PA x-ray film | 0.02 |
| Lumbar spine | 1.8 |
| KUB abdomen | 0.53 |
| CT abdomen | 10 |
| CT chest | 20-40 |
| PET-CT | 25 |
| Ultrasound or MRI | 0 |
Table 5-2
Suggested imaging in renal disease.
These recommendations assume availability of all common imaging modalities. CT, Computed tomography; CTA, computed tomographic angiography; MRA, magnetic resonance angiography. (Modified from reference 1.)
| Recommended Imaging in Renal Disease | |
| Renal Pathology | First-Choice Imaging |
| Acute kidney injury, chronic kidney disease | Ultrasound |
| Hematuria | Ultrasound or CT |
| Proteinuria, nephrotic syndrome | Ultrasound CT urography |
| Hypertension with normal renal function | Ultrasound Consider CTA or MRA |
| Hypertension with impaired renal function | Ultrasound with Doppler |
| Renal infection | Contrast-enhanced CT |
| Hydronephrosis identified on ultrasound | Nuclear renogram |
| Retroperitoneal fibrosis | Contrast-enhanced CT |
| Papillary or cortical necrosis | Contrast-enhanced CT |
| Renal vein thrombosis | Contrast-enhanced CT |
| Renal infarction | Contrast-enhanced CT |
| Nephrocalcinosis | CT |
Table 5-3
Risk estimates in diagnostic imaging.
| Risk Estimates in Diagnostic Imaging | |
| Imaging Risk | Estimated Risk |
| Cancer from 10 mSv of radiation (1 body CT)3 | 1 in 1000 |
| Contrast-induced nephropathy in patient with renal impairment4 | 1 in 5 |
| Nephrogenic systemic fibrosis5,6 | 1 in 25,000 to 1 in 30,000 (depends on gadolinium agent) 1 in 5 if GFR <30/ml |
| Death from iodine contrast anaphylaxis7 | 1 in 130,000 |
| Death from gadolinium contrast anaphylaxis8 | 1 in 280,000 |
Ultrasound
Ultrasound is relatively inexpensive and provides a rapid way to assess renal location, contour, and size without radiation exposure. Nephrologists are increasingly undertaking straightforward ultrasound examination; the practical techniques as well as the appropriate interpretative skills are discussed in Chapter 92. Portable ultrasound is available and is essential in the pediatric or emergency setting. Obstructing renal calculi can be readily detected, and renal masses can be identified as cystic or solid. In cases of suspected obstruction, the progression or regression of hydronephrosis is readily evaluated. Color Doppler imaging permits assessment of renal vascularity and perfusion. Unlike the other imaging modalities, ultrasound is highly dependent on operator skills. Limitations of ultrasound include lack of an acoustic window, body habitus, and poor patient cooperation.
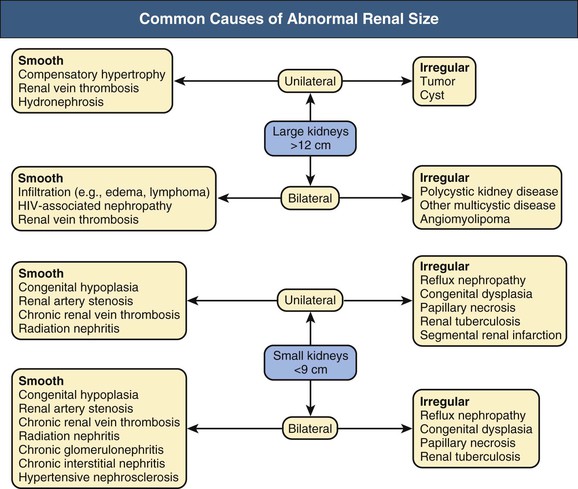
Kidney Size
The kidney is imaged in transverse and sagittal planes and is normally 9 to 12 cm in length in adults. Differences in kidney size can be detected with all imaging modalities. Figure 5-1 diagrams the common causes of enlarged and shrunken kidneys.
Renal Echo Pattern
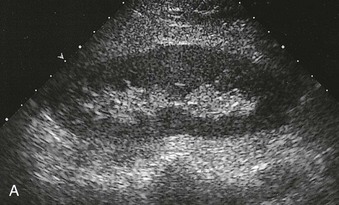
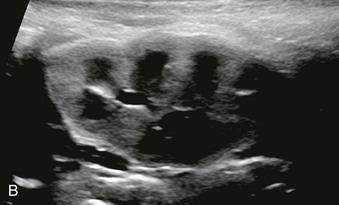
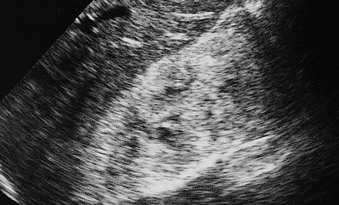
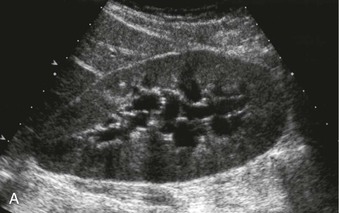
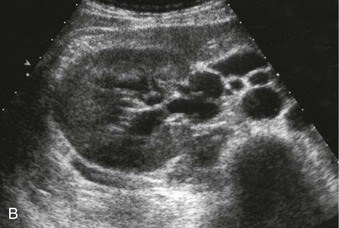
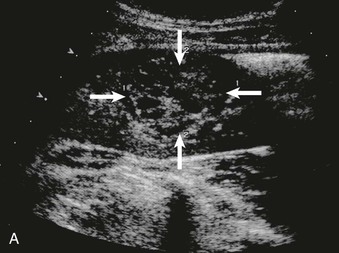
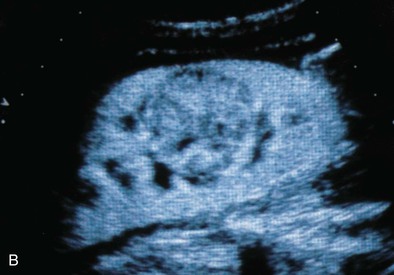
The normal renal cortex is hypoechoic compared with the fat-containing echogenic renal sinus (Fig. 5-2, A). The cortical echotexture is defined as isoechoic or hypoechoic compared with the liver or spleen. In children, the renal pyramids are hypoechoic (Fig. 5-2, B), and the cortex is characteristically hyperechoic compared with the liver and the spleen.2 In adults, an increase in cortical echogenicity is a sensitive marker for parenchymal renal disease but is nonspecific (Fig. 5-3). Decreased cortical echogenicity can be found in acute pyelonephritis and acute renal vein thrombosis.
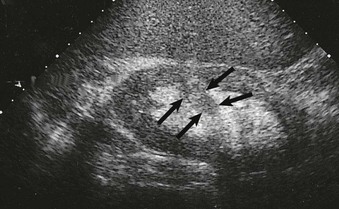
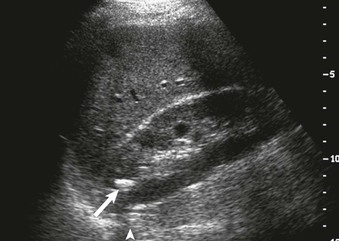
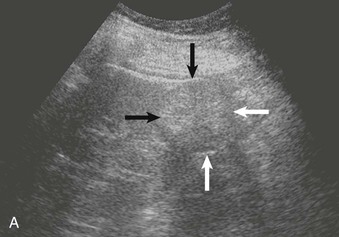
The normal renal contour is smooth, and the cortical mantle should be uniform and slightly thicker toward the poles. Two common benign pseudomasses that can be seen with ultrasound are the dromedary hump and the column of Bertin. The column of Bertin results from bulging of cortical tissue into the medulla; it is seen as a mass with an echotexture similar to that of the cortex, but it is found within the central renal sinus (Fig. 5-4). The renal pelvis and proximal ureter are anechoic. An extrarenal pelvis refers to the renal pelvis location outside the renal hilum. The ureter is not identified beyond the pelvis in nonobstructed patients.
Obstruction can be identified by the presence of hydronephrosis (Fig. 5-5). Parenchymal and pelvicalyceal nonobstructing renal calculi as well as ureteral obstructing calculi can be readily detected (Fig. 5-6). The upper ureter will also be dilated if obstruction is distal to the pelviureteral junction (see Fig. 5-5, C). False-negative ultrasound examination findings with no hydronephrosis occasionally occur in early obstruction. Obstruction without ureteral dilation may also occur in retroperitoneal fibrosis and in transplanted kidneys as a result of periureteral fibrosis.
Renal Cysts
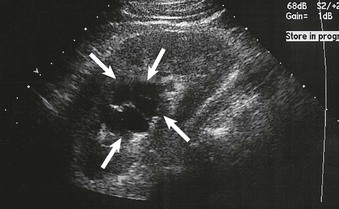
Cysts can be identified as anechoic lesions and are a frequent coincidental finding during renal imaging. Ultrasound usually readily identifies renal masses as cystic or solid (Figs. 5-7 and 5-8). However, hemorrhagic cysts may be mistakenly called solid because of increased echogenicity. Differentiation of cysts as simple or complex is required to plan intervention.
Simple Cysts
A simple cyst on ultrasound is anechoic, has a thin or imperceptible wall, and demonstrates through-transmission because of the relatively rapid progression of the sound wave through fluid compared with adjacent soft tissue.
Complex Cysts
Complex cysts contain calcifications, septations, and mural nodules. Instead of being anechoic, these masses may contain internal echoes representing hemorrhage, pus, or protein. Complex cysts may be benign or malignant; cyst wall nodularity, septations, and vascularity strongly suggest malignancy. The Bosniak classification of cystic renal masses is widely used (see Table 61-5). Complex cysts identified by ultrasound require further evaluation by contrast-enhanced CT (or MRI) to identify abnormal contrast enhancement of the cyst wall, mural nodule, or septum.
Bladder
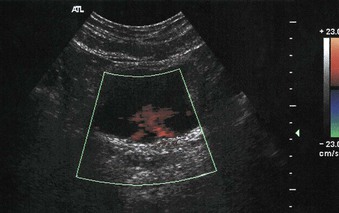
Real-time imaging can be used to evaluate for bladder wall tumors and bladder stones. Color flow Doppler evaluation of the bladder in well-hydrated patients can be used to identify a ureteral jet, produced when peristalsis propels urine into the bladder. The incoming urine has a higher specific gravity relative to the urine already in the bladder (Fig. 5-9). Absence of the ureteral jet can indicate total ureteral obstruction.
Renal Vasculature
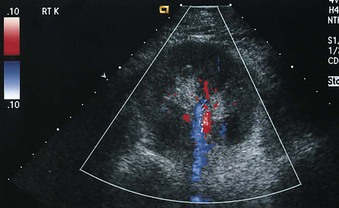
Color Doppler investigation of the kidneys provides a detailed evaluation of the renal vascular anatomy. The main renal arteries can be identified in most patients (Fig. 5-10). Power Doppler imaging is a more sensitive indicator of flow, but unlike color Doppler imaging, power Doppler provides no information about flow direction and cannot be used to assess vascular waveforms. However, power Doppler imaging is exquisitely sensitive for detection of renal parenchymal flow and has been used to identify cortical infarction.
Renal Artery Duplex Scanning
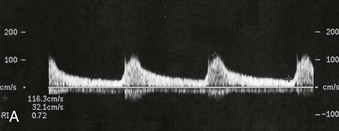
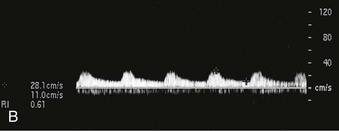
The role of gray-scale and color Doppler sonography in screening for renal artery stenosis is controversial. The principle is that a narrowing in the artery will cause a velocity change commensurate with the degree of stenosis as well as a change in the normal renal artery waveform downstream from the lesion. The normal renal artery waveform demonstrates a rapid systolic upstroke and an early systolic peak (Fig. 5-11, A). The waveform becomes dampened downstream from a stenosis. This consists of a slow systolic acceleration (tardus) and a decreased and rounded systolic peak (parvus) (Fig. 5-11, B). It also results in a decrease in the resistive index, defined as the end-diastolic velocity (EDV) subtracted from the peak systolic velocity (PSV) divided by PSV: (PSV − EDV)/PSV. The normal resistive index is 0.70 to 0.72.
The entire length of the renal artery should be examined for the highest velocity signal. The origins of the renal arteries are important to identify because this area is often affected by atherosclerosis, but the arteries are often difficult to visualize because of overlying bowel gas. Within the kidney, medullary branches and cortical branches in the upper, middle, and lower thirds should be included to attempt detection of stenosis in accessory or branch renal arteries.
Proximal and distal criteria exist for diagnosis of significant renal artery stenosis, usually defined as stenosis greater than 60%. The proximal criteria detect changes in the Doppler signal at the site of stenosis and provide sensitivities and specificities ranging from, respectively, 0% to 98% and 37% to 98%.9,10 Technical failure rates are typically 10% to 20%.11 Renal artery stenosis may also be missed if PSV is low because of poor cardiac output or aortic stenosis. False-positive results can occur when renal artery velocity is increased because of high-flow states, such as hyperthyroidism or vessel tortuosity.12 The distal criteria are related to detection of a tardus-parvus waveform distal to a stenosis; sensitivities and specificities of 66% to 100% and 67% to 94%, respectively, have been reported.13,14 Technical failure with distal criteria is much lower than with proximal evaluation (<5%). False-negative results can occur from stiff poststenotic vessels, which will decrease the tardus-parvus effect.15 The tardus-parvus effect may also be a result of aortic stenosis, low cardiac output, or collaterals in complete occlusion, giving a false-positive result.
Combining the proximal and distal criteria improves the detection of stenoses. Sensitivity of 97% and specificity of 98% can be achieved when both the extrarenal and the intrarenal arteries are examined.16 When it is technically successful, Doppler ultrasound has a negative predictive value of more than 90%.16 However, reliable results require a skilled and experienced sonographer and a long examination time. Despite these limitations, Doppler studies also have several advantages. Noninvasive, inexpensive, and widely available, Doppler studies also allow structural and functional assessment of the renal arteries and imaging without exposure to radiation or nephrotoxic agents.
Some physicians prefer CT angiography (CTA) or magnetic resonance angiography (MRA) as a faster and more reliable screening tool than ultrasound, but at present the choice should depend on local expertise and preference. For further discussion of the diagnosis and management of renovascular disease, see Chapters 39 and 66.
Contrast-Enhanced and Three-Dimensional Ultrasound
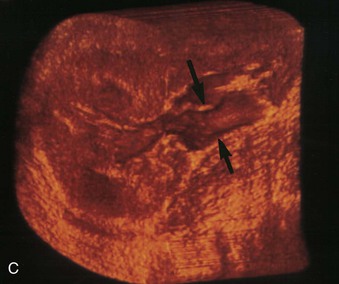
Ultrasound contrast agents, initially introduced to assess cardiac perfusion, are now being used to evaluate perfusion to other organs, such as the kidney. These intravenous agents are microbubbles 1 to 4 µm in diameter (smaller than erythrocytes) that consist of a shell surrounding the echo-producing gas core. The microbubbles oscillate in response to the ultrasound beam frequency and give a characteristic increased echo signal on the image. Preliminary studies evaluating renal perfusion in dysfunctional kidneys show reduced flow compared with normal kidneys as well as improved lesion detection (Fig. 5-12). However, the clinical adoption of microbubble imaging in the kidney remains uncertain, particularly with the general availability and robustness of CT and MRI, and its use is off-label in the United States and not presently reimbursed.
Two-dimensional ultrasound images can be reconstructed into three-dimensional (3D) volume images by a process similar to 3D reconstructions for MRI and CT. Although the current techniques are time-consuming, technical improvements should decrease 3D reconstruction time. Potential applications include vascular imaging and fusion with MRI or positron emission tomography (PET).
Plain Radiography and Intravenous Urography
The use of intravenous urography (IVU) has receded as cross-sectional imaging by CT or MRI has become more widely applied to the urinary tract. Although now with few primary indications in many centers, contrast urography may still be a key investigation in parts of the world where economic limitations mean that cross-sectional imaging is not available. However, plain radiography, often called a KUB (kidneys, ureter, bladder), still has an important role in the identification of soft tissue masses, bowel gas pattern, calcifications, and renal location.
Renal Calcification
Most renal calculi are radiodense, although only about 60% of urinary stones detected on CT are visible on plain films.17 CT demonstrates nonopaque stones, which include uric acid, xanthine, and struvite stones. However, neither CT nor plain radiography may detect calculi associated with protease inhibitor therapy.18 Oblique films are sometimes obtained to confirm whether a suspicious upper quadrant calcification is renal in origin. Calculi that are radiolucent on plain films are usually detected as filling defects on IVU. IVU has higher sensitivity than radiography but lower sensitivity than CT. If available, CT is the imaging modality of choice for detection of urinary calculi.19
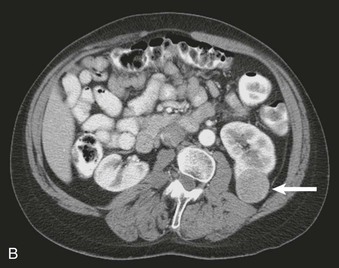
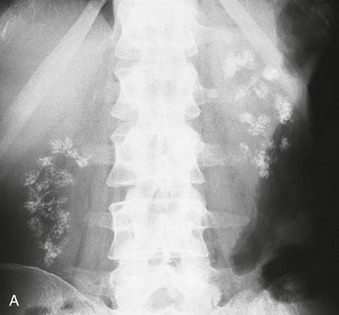
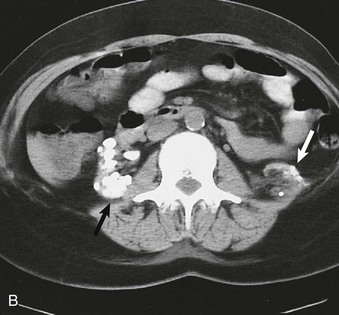
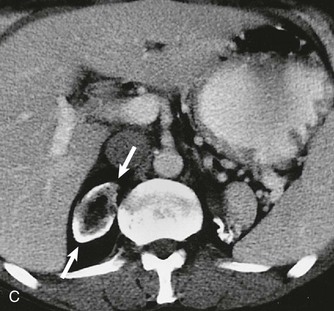
Nephrocalcinosis may be medullary (Fig. 5-13, A and B) or cortical (C) and is localized or diffuse. The common causes of nephrocalcinosis are discussed in Chapter 59 (see Box 59-7).
Intravenous Contrast Urography
Before contrast material is administered, an abdominal compression device may be placed, to compress the midureters against the bony pelvis. This retains the excreted contrast material in the upper tract and distends the renal pelvis and calyces. The first film is usually performed at 30 seconds after contrast injection, when the renal parenchyma is at peak enhancement. Subtle renal masses are often detected only on these early films. The compression device is then removed, and films of the entire abdomen are obtained at 5 minutes, when there is renal excretion of the contrast agent and the ureters are best evaluated. Films with the patient prone may be required to visualize the entirety of the ureter. A filled-bladder film is obtained. A postvoid film of the bladder assesses emptying and assists in evaluation of the distal ureters, which may be obscured by a distended contrast-filled bladder. The usual contrast volume injected for IVU is similar to that for routine abdominal CT. The primary difference is timing of imaging. IVU is contraindicated in patients with a history of allergic reactions to radiographic contrast agents. When the glomerular filtration rate (GFR) is less than 60 ml/min, IVU yields increasingly poor images, and the risk of nephrotoxicity also increases.
Kidneys
Evaluation of the kidneys on IVU (as well as CT and MRI) should include their number, location, axis, size, contour, and degree of enhancement. Renal size is variable, but a normal kidney should be about three to four lumbar vertebral bodies in length. The renal outline should be smooth and sharply demarcated from the retroperitoneal fat. Renal enhancement after contrast administration should be symmetric and progress centrally from the cortex, with excretion evident in the ureters by 5 minutes. Asymmetry of renal enhancement may indicate renal artery disease.
Pelvicalyceal System
The pelvicalyceal system is best evaluated on the early postcontrast films. Normally, there are about 10 to 12 calyces per kidney. The calyces drain into the infundibula, which in turn empty into the renal pelvis (Fig. 5-14). The infundibulum and renal pelvis should have smooth contours without filling defects. In a common variant, vessels can cross the pelvicalyceal system or ureters, causing extrinsic compression defects that should not be mistaken for tumors or other urothelial lesions. When more than one calyx drains into an infundibulum, it is known as a compound calyx, most frequently seen in the poles. The normal calyx is gently cupped. Calyceal distortion occurs with papillary necrosis and reflux nephropathy.
Ureters
The ureters are often seen segmentally because of active peristalsis. The ureters should be free of filling defects and smooth. In the abdomen, the ureters lie in the retroperitoneum, passing anterior to the transverse processes of the vertebral bodies. In the pelvis, the ureters course laterally and posteriorly, eventually draining into the posteriorly located vesicoureteral junction. At the vesicoureteral junction, the ureters gently taper. Medial bowing or displacement of the ureter is often abnormal and can be seen secondary to ureter displacement from retroperitoneal masses, lymphadenopathy, and retroperitoneal fibrosis.
Bladder
The bladder should be rounded and smooth walled. Benign indentations on the bladder include the uterus, prostate gland, and bowel. In chronic bladder outlet obstruction and neurogenic bladder, numerous trabeculations and diverticula may be seen around the bladder outline.
Retrograde Pyelography
Retrograde pyelography is performed when the ureters are poorly visualized on other imaging studies or when samples of urine need to be obtained from the kidney for cytology or culture. Patients who have severe allergies to contrast agents or impaired renal function can be evaluated with retrograde pyelography. The examination is performed by placing a catheter through the ureteral orifice under cystoscopic guidance and advancing it into the renal pelvis. With use of fluoroscopy, the catheter is slowly withdrawn while radiocontrast is injected (see Figs. 60-4 and 60-13). This technique provides excellent visualization of the renal pelvis and ureter and can also be used for cytologic sampling from suspect areas.
Antegrade Pyelography
Antegrade pyelography is performed through a percutaneous renal puncture and is used when retrograde pyelography is not possible. Ureteral pressures can be measured, hydronephrosis evaluated, and ureteral lesions identified (see Fig. 60-16). The examination is often performed as a prelude to nephrostomy placement. Both antegrade pyelography and retrograde pyelography are invasive and should be performed only when other studies are inadequate.