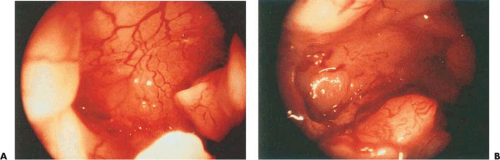
Hysteroscopy in Gynecologic Malignancy
Michael S. Baggish
Hubert Guedj
Premalignant and Malignant Lesions of the Cervix
Although previous data have been reported about the adjunct use of hysteroscopy in the diagnosis of premalignant lesions of the cervix, the practicality of this application is nebulous. Although the second edition of this book included details of microcolpohysteroscopy, the technique was minimally used and never really caught on with the gynecologic community. In fact, the technique of microcolpohysteroscopy was in the main championed by only a handful of gynecologists who were mainly based in Europe and included J. E. Hamou and L. Mencaglia, whom we have referenced in this chapter.
The main value of either contact or panoramic hysteroscopy is to assess the blind spot of colposcopy, i.e., the endocervical canal. To determine the distance and location of the extension of the abnormal transformation zone into the endocervical canal, a 3-mm office hysteroscope or 6-mm contact hysteroscope may be inserted into the cervical canal that has been doused with 4% acetic acid. The white epithelium may then be followed upward by direct-view hysteroscopy. This technique has the advantage of not only directing endocervical sampling (curettage) but also quantifying the height of a loop electrical excision or knife conization.
The second application for cervical canal endoscopy is the localization and detection of primary adenocarcinoma of the endocervical canal as well as metastatic adenocarcinoma emanating from a primary corpus cancer. Hysteroscopic examination of the endocervical canal is prompted by AGUS (atypical glandular cells of undetermined significance cytology). Hysteroscopy adds a sight dimension to an otherwise blind endocervical curettage. Unless the cervix is stenotic, this endoscopic procedure may be easily performed using a 3-mm telescope and a 4-mm sheath with distension provided by Hyskon and delivered via a Cook hand pump. In most circumstances, 50 mL of Hyskon will be sufficient for this simple inspection. Obviously, lesions buried beneath the cervical epithelium, i.e., lying deep with the stroma, will not be seen by hysteroscopic examination (Figs. 23.1 and 23.2).
Premalignant and Malignant Lesions of the Endometrial Cavity
The current techniques leading the practitioner to the timely diagnosis of endometrial cancer include: cytology, endometrial biopsy, dilatation and curettage, transvaginal ultrasound scanning, hysterosonography, and hysteroscopy.
The great majority, i.e., three-fourths of endometrial carcinomas, make their presence known at an early phase of the disease. The symptom that alerts the patient to its presence is abnormal uterine bleeding, and the most prevalent age group comprises those women older than 50 years of age. Therefore, women who report postmenopausal bleeding require a workup to determine the source of the bleeding. The severity of blood loss by this route varies from intermenstrual spotting to sustained menorrhagia. Although articles have been published about “emergency hysteroscopy,” the justification for a bona fide emergent hysteroscopic operation has been elusive. Shalev et al. (2004) reported on 40 women who underwent emergency hysteroscopy. The major focus of the study centered around endometrial resection as the emergency technique for the management of uterine bleeding. One case of adenocarcinoma was picked up via the resected “chips.” The author considers that there are few if any indications for “emergency” hysteroscopy. Clearly, an accurate diagnosis should precede any precipitous hysteroscopic enterprise.
Endometrial height or thickening may be accurately established by timed transvaginal ultrasound imaging. The technique may reasonably suggest to the clinician that hyperplasia or carcinoma is present or absent. If a benchmark for endometrial thickness is set at ≤4 mm, then the sensitivity for ruling out endometrial cancer or hyperplasia is ≥90%. If the normal thickness cutoff is 3 mm, then the sensitivity of the test is approximately 99%. However, the gynecologist must also bear in mind that certain varieties of
endometrial carcinoma may not be associated with a thickened endometrium. Papillary serous adenocarcinoma of the endometrium may have ultrasound findings of 3 to 4 mm endometrial thickness yet are quite lethal, particularly if the diagnosis is delayed.
endometrial carcinoma may not be associated with a thickened endometrium. Papillary serous adenocarcinoma of the endometrium may have ultrasound findings of 3 to 4 mm endometrial thickness yet are quite lethal, particularly if the diagnosis is delayed.
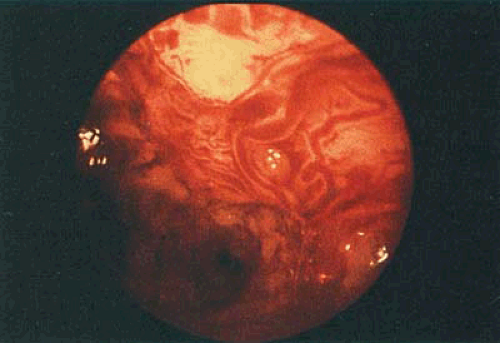
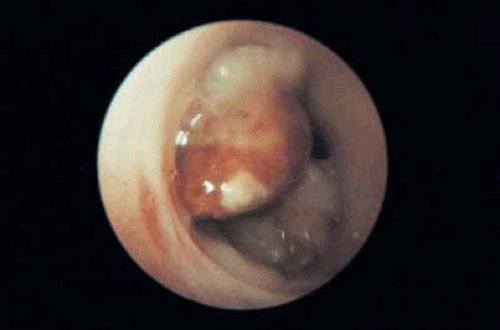 FIGURE 23.1 Fleshy, polypoidal adenocarcinoma of the endocervical canal spreading into the uterine isthmus. |
Benign endometrial hyperplasia includes both simple and complex forms and is apt to be associated with dysfunctional bleeding and hyperestrogenic states. From a hysteroscopic point of view, these cases often present a picture similar to that of a variant of normal endometrium. The plasticity of the mucosa makes it possible to estimate its thickness by means of the pressure of the endoscope on the uterine wall, resulting in an indentation (Fig. 23.3A). The cystic form often displays a characteristic hysteroscopic picture of raised glandular orifices with real cystic formations of about a millimeter in diameter. The same formation may be found in an endometrium of reduced thickness, indicating cystic atrophy. Apart from the cystic form, which presents specific aspects, the hysteroscopic changes seen in benign endometrial hyperplasia are varied and often multiple. The following are some of the more commonly found hysteroscopic aspects (Fig. 23.3B):
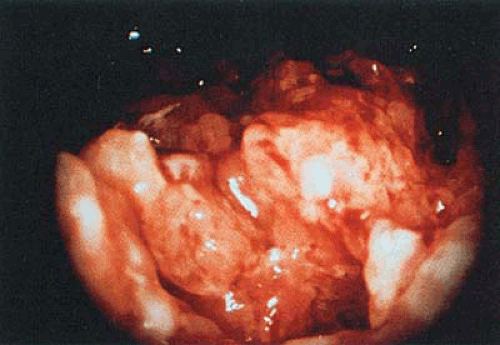
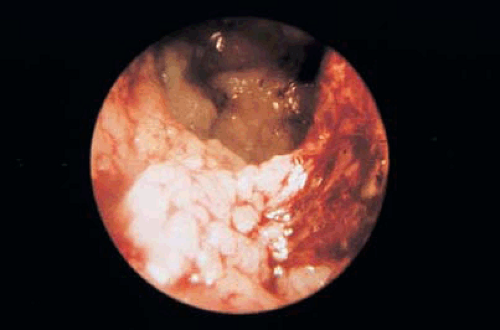 FIGURE 23.2 Endometrial cancer has spread to the endocervix. Note the cerebriform mass on the right and the surface extension of the cancer on the left under the internal cervical os. |
Increased endometrial thickness
Nonhomogeneous endometrial regeneration
Increased vascularization
Presence of ciliate images
Presence of cystic dilatations
Increased bleeding
Polypoid formations
Necrotic areas
Irregular arrangement and concentration of the glandular orifices
If one or more of these elements are found, the endoscopist should suspect the presence of benign endometrial hyperplasia, and a directed biopsy should be performed (Fig. 23.4).
Atypical endometrial hyperplasia is also known as carcinoma in situ of the endometrium. The hysteroscopic and histologic features are more comparable with neoplastic lesions. In these situations the hysteroscopic picture is also extremely varied: There is substantial architectural upheaval, polypoid growth is often present, and vascularization is clearly visible and pathologic. Superficial capillary vascularization takes on a bizarre configuration, sometimes exhibiting a corkscrew pattern where the vessels surround groups of glandular openings (Fig. 23.5). In such cases the endoscopist must immediately confirm the hysteroscopic diagnosis by means of an endometrial biopsy.
In a previous study, we attempted to assess the diagnostic accuracy of the hysteroscopic appearance alone compared with the histologic diagnosis. The hysteroscopic diagnosis of endometrial hyperplasia in 98 patients agreed completely with the histology results in 70% of the cases, whereas in 30% a false-positive diagnosis of endometrial hyperplasia was made when in reality normal or atrophic endometrium existed. Out of 12 patients with the hysteroscopic diagnosis of atypical hyperplasia, histologic confirmation was obtained in 11 cases (92%). In one case, atrophic endometrium was diagnosed histologically.
Adenocarcinoma of the endometrium is clearly the most significant disorder requiring timely and accurate diagnosis. Hysteroscopy has proven to be an extremely reliable method for the diagnosis of this group of diseases. The endoscopic patterns of endometrial cancer are reasonably obvious and are rarely confused with other lesions. In its initial stage the adenocarcinoma shows a germinative picture, with irregular, polylobate, friable projections that are usually necrotic and bleed easily (Figs. 23.6 and 23.7). Vascularization is irregular and bizarre (Fig. 23.8). In some cases a distinct zone between the neoplasia and the normal endometrium can be seen. It is sometimes possible to see focal lesions, often in the cornu where they could easily be missed with the use of
blind sampling techniques. The macroscopic view of the endometrium can correlate with changes in the glandular structure, which are likewise observed by microscopic examination. Seventy percent of our cases were categorized as stage I disease (i.e., limited to the uterine corpus) (Figs. 23.9 and 23.10). Spread to the cervical canal (Fig. 23.11) was identified hysteroscopically with high accuracy. In 17 patients with confirmed histologic diagnosis of endometrial neoplasia in the cervix, hysteroscopic diagnosis agreed in 94.2% of cases (see Figs. 23.9, 23.10, 23.11).
blind sampling techniques. The macroscopic view of the endometrium can correlate with changes in the glandular structure, which are likewise observed by microscopic examination. Seventy percent of our cases were categorized as stage I disease (i.e., limited to the uterine corpus) (Figs. 23.9 and 23.10). Spread to the cervical canal (Fig. 23.11) was identified hysteroscopically with high accuracy. In 17 patients with confirmed histologic diagnosis of endometrial neoplasia in the cervix, hysteroscopic diagnosis agreed in 94.2% of cases (see Figs. 23.9, 23.10, 23.11).
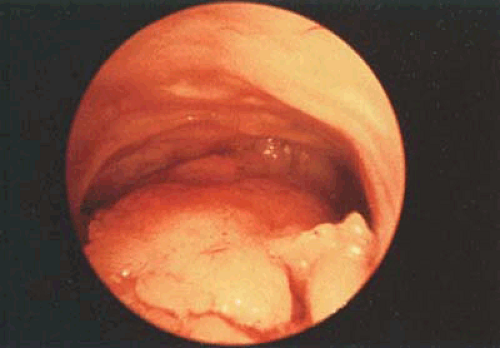 FIGURE 23.4 Polypoid hyperplasia located on the posterior wall; the rest of the endometrial mucosa is atrophic. In this case it is very easy to miss the lesion with a blind sampling technique. |
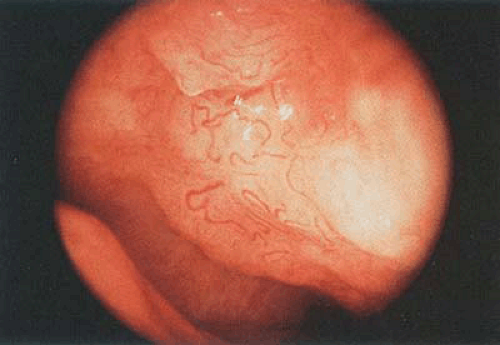 FIGURE 23.5 Close-up hysteroscopic observation showing atypical vascularization associated with adenomatous hyperplasia. |
Although the diagnostic accuracy of hysteroscopy was extremely high, it should not be considered an end unto itself but rather as a portion of the investigation to use in concert with endometrial biopsy. Hysteroscopy can be used for the selection of those patients who are at risk for malignant disease of the endometrium as well as to help direct the biopsy to establish an accurate diagnosis. In the same vein, hysteroscopy is a useful tool for excluding further investigations in those patients who show no evidence of disease. The number of cases in which hysteroscopy observation is sufficient, without the help of a subsequent biopsy for reaching a diagnosis, will depend directly on the endoscopist’s experience. It is possible, even after substantial practice in the use of hysteroscopy, to identify patients suffering from benign or malignant endometrial disease with a 20% rate of false-positive but no false-negative diagnoses. It is therefore clear that the association of hysteroscopy and biopsy can approach 100% sensitivity in the diagnosis of endometrial neoplasia and its precursors. The technical features of the method, shown in Table 23.1, illustrate that a combination of hysteroscopy and directed biopsy offer the best technique for the early detection of endometrial neoplasia and its precursors.
Diagnosis by Hysteroscopy
Marchetti et al. (2002) examined the role of hysteroscopy in the early diagnosis of endometrial cancer by comparing the disease-free survival of 181 proven endometrial cancer cases. Patients were divided based on those who underwent hysteroscopy versus no hysteroscopy. In the series, the authors demonstrated high accuracy for the hysteroscopic diagnosis of endometrial cancer (93% sensitivity, 99.9% specificity). Stage IA cases were diagnosed in 23% of hysteroscopy cases versus 15% on nonhysteroscopy. Survival was 100% and 91.7% at 3 and 5 years, respectively.
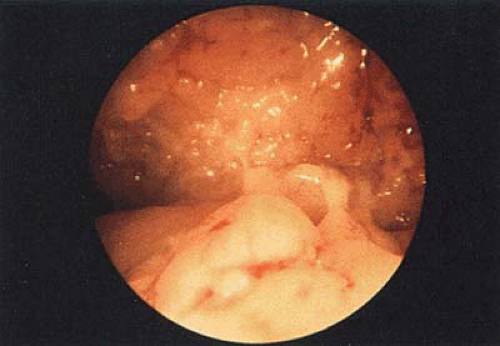 FIGURE 23.6 Panoramic picture of a well-differentiated adenocarcinoma of the endometrium. Note the cerebroid pattern typical of this lesion. |
Clark et al. (2002) published a meta-analysis to determine the accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia. The study found that compared with prehysteroscopy probability of 3.9% for cancer, a positive hysteroscopy increased the probability to 71.8% whereas a negative hysteroscopy reduced the probability of cancer to 0.6%.
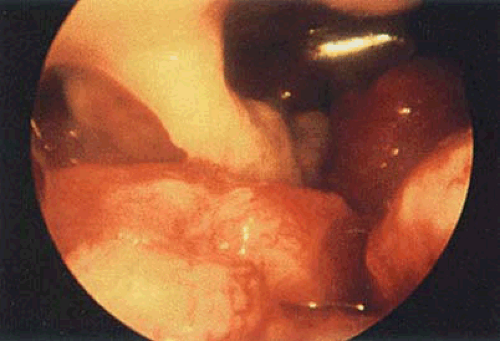 FIGURE 23.7 Panoramic vision of a completely altered uterine architecture in a patient with undifferentiated adenocarcinoma of the endometrium. |
Bain et al. randomized women to hysteroscopy and endometrial biopsy versus endometrial biopsy alone. Based on 370 women studied, the authors concluded that hysteroscopy would be most useful in selected cases rather than be performed in a nonselective manner. Ben-Yehuda et al. and De Wit et al. came to similar conclusions.
As with colposcopy, hysteroscopy requires more skill and training than does simple endometrial biopsy. When an appropriately trained hysteroscopist performs a directed biopsy after identifying an area within the uterine cavity as suspicious for malignancy or hyperplasia, then the accuracy
of the biopsy should be increased. No urologist would consider blind sampling of the bladder to rule in or out malignant disease. It remains a mystery why gynecologists continue to embrace blind procedures to make critical diagnoses. Studies that compare hysteroscopic view must be accompanied by a sampling. Cancer and hyperplasia can be a suspected hysteroscopic impression, but the diagnosis must be made based on sampling and histopathologic criteria. Logic would require explanation if a uterine cavity was completely normal by hysteroscopy and a blind biopsy turned up adenocarcinoma of the endometrium. Even the optically unaided gross examination of a hysterectomy specimen in the pathology laboratory will find the most likely site for the suspected cancer and focus the sampling for sections at that location.
of the biopsy should be increased. No urologist would consider blind sampling of the bladder to rule in or out malignant disease. It remains a mystery why gynecologists continue to embrace blind procedures to make critical diagnoses. Studies that compare hysteroscopic view must be accompanied by a sampling. Cancer and hyperplasia can be a suspected hysteroscopic impression, but the diagnosis must be made based on sampling and histopathologic criteria. Logic would require explanation if a uterine cavity was completely normal by hysteroscopy and a blind biopsy turned up adenocarcinoma of the endometrium. Even the optically unaided gross examination of a hysterectomy specimen in the pathology laboratory will find the most likely site for the suspected cancer and focus the sampling for sections at that location.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree