Hysteroscopic Myomectomy
Rafael F. Valle
Michael S. Baggish
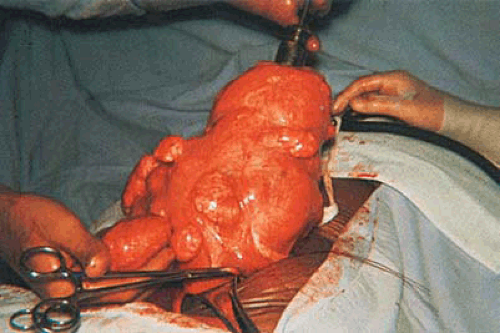
Uterine leiomyomas are benign solid tumors occurring in about 20% to 30% of women during their reproductive years (Fig. 26.1). Submucous myomas may present a greater risk to the patient than either the intramural or subserous varieties because they cause excessive uterine bleeding, usually during menses, and they can cause dysmenorrhea as well as interfere with normal reproductive mechanisms. Submucous myomas characteristically are associated with chronic endometritis, may have a greater risk for malignant change (leiomyosarcoma), and are prone to bleed without provocation. Since these tumors frequently cannot be palpated during examinations or felt during blind intrauterine manipulations, they may go undetected until the abnormal bleeding is severe enough to cause anemia. Methods used to evaluate these tumors are hysterosalpingography (HSG); magnetic resonance imaging (MRI); sonography, particularly with vaginal probes; and direct visualization of the uterine cavity by hysteroscopy. With improvements in instruments designed for visualizing the uterine cavity and performing transcervical surgery, symptomatic tumors have been approached transcervically, avoiding hysterotomies and concomitant laparotomy.
Biology
All myomas are parasitic, obtaining their vasculature and sucking the blood supply from the surrounding myometrium. Characteristically, the arterial supply to an individual myoma is limited and accounts for the high incidence of chronic muscle ischemia, which is evidenced by hyalinization. The major vascular abnormalities accounting for the propensity to bleed result from the abnormal venous drainage. Several studies have identified numerous aberrant, thin-walled, venous channels associated with uterine myomas.
Uterine leiomyomas arise from the smooth muscle cells of the uterus or from the media of myometrial arterioles. As they grow, they usually migrate to a place of less resistance, i.e., toward the abdominal cavity, becoming subserous or following the path of the intrauterine cavity and becoming submucous. Most intracavitary tumors have a portion of the tumor submerged within the uterine wall and are termed sessile submucous leiomyomas. In contrast, those that have a stalk or pedicle are termed pedunculated. These tumors vary in size from <1 cm to >10 cm. Because these tumors usually lack a normal endometrium, the thin-walled, sinusoidal surface vessels can easily be observed under hysteroscopic view. If the vessel wall is breech, a burst of blood can be seen erupting from these vessels, which results from a lack of self-hemostatic, i.e., vascular mechanisms. The result is the rapid filling of the uterine cavity with blood.
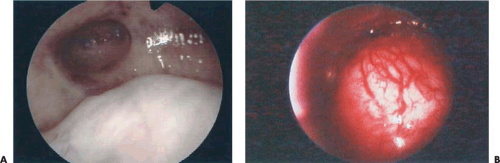
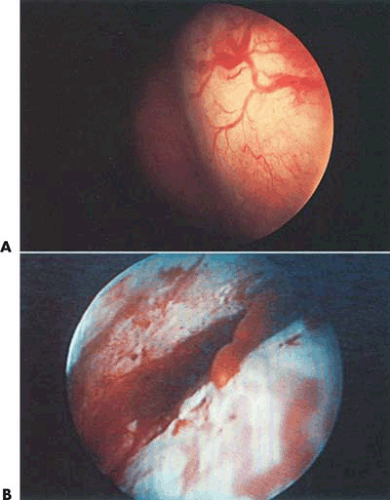
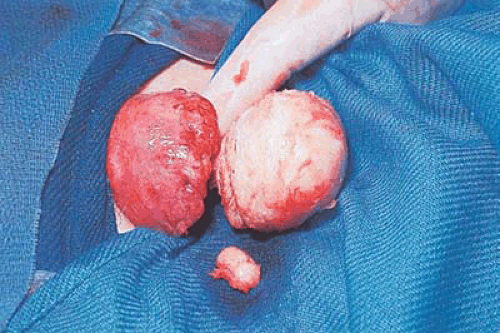
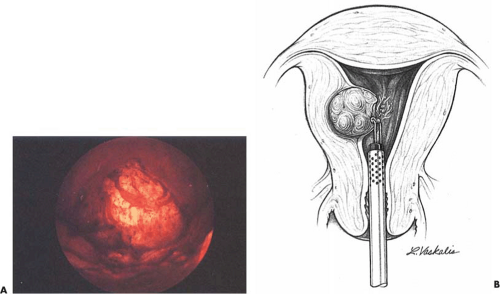
Some tumors demonstrate a small portion protruding into the uterine cavity analogous to the tip of an iceberg (Fig. 26.2). Removal of mainly intramural tumors requires a transabdominal approach by hysterotomy (Fig. 26.3). Other myomas protrude completely into the uterine cavity (pedunculated), and still others have a portion of their body penetrating the uterine wall (Figs. 26.4 and 26.5A). Based on these observations, these myomas were classified into the following three types: type 0 pedunculated myomas, not involving the myometrium; type 1 with <50% myometrial involvement; and type 2 with >50% myometrial penetration.
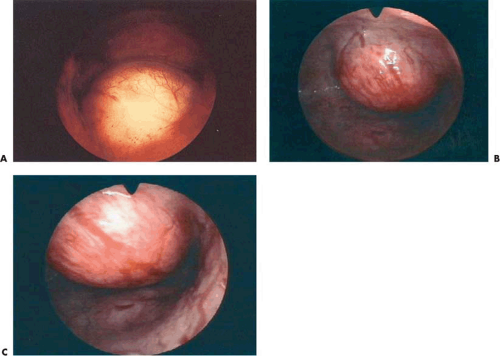
Most submucous tumors occur within the corpus of the uterine cavity. Some are fundal, whereas others are anteriorly, posteriorly, or laterally situated. Small tumors may also arise at the cornual regions, interfering with the uterotubal junction lumen. A few are located in the cervical canal or within the close vicinity of the internal cervical os (Fig. 26.5B).
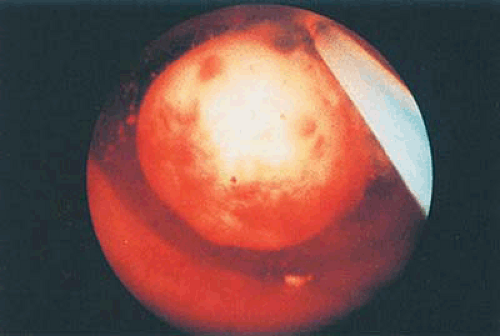
The principal reason for treating submucous myomas is abnormal uterine bleeding (Fig. 26.6). Secondary indications are infertility, pregnancy wastage, and less commonly pain. For patients in their reproductive years who desire fertility, myomectomy is the treatment of choice to preserve the uterus. Should other uterine myomas contribute to symptomatology, additional concomitant surgery may be required (Fig. 26.7). In women who do not desire further childbearing and have only submucous leiomyomas, the transcervical approach is also appropriate, if hysterectomy is not desired. Should other associated pathologic conditions require treatment, a hysterectomy must be seriously considered. The decision to approach a leiomyoma by myomectomy in a patient who has completed her childbearing therefore requires clinical judgment, patient counseling, and a clear understanding of the risk–benefit ratio.
Prior to operative treatment, those patients who are anemic or bleeding excessively should be prepared for a minimum of 3 months with gonadotropin-releasing hormone
(GnRH) agonist. This will shrink the myoma and also reduce its blood supply. The dosage of GnRH agonist ranges (as noted in the chapters on preparation, drugs, and ablation) is from 3.60 mg to 3.75 mg monthly.
(GnRH) agonist. This will shrink the myoma and also reduce its blood supply. The dosage of GnRH agonist ranges (as noted in the chapters on preparation, drugs, and ablation) is from 3.60 mg to 3.75 mg monthly.
Operative Hysteroscopic Methods
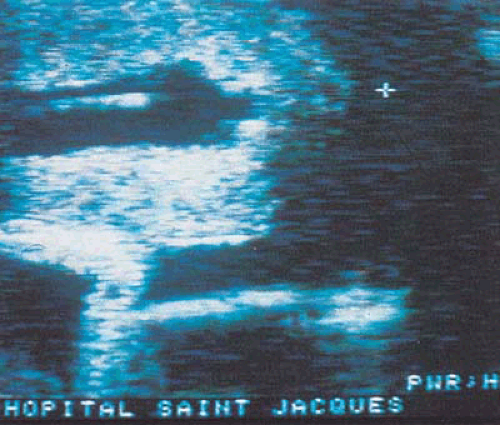
Large myomas treated by hysteroscopy may require simultaneous laparoscopy. A recently published alternative to simultaneous laparoscopic monitoring of operative hysteroscopy is real-time ultrasonography (Fig. 26.8). One technique describes three contrasts obtained by (i) filling the urinary bladder with fluid, (ii) infusing the cul-de-sac by gravity, and (iii) distending the uterine cavity with fluid and inserting an operative device. Essentially, real-time ultrasonography provides an indirect visual image of the uterine wall, the lesions, and the hysteroscope. Neuwirth and Amin were pioneers in developing techniques for the hysteroscopic treatment of submucous myomas. Initially the lesions were removed by rather rugged methods (e.g., ovum forceps were used to twist pedunculated myomas off their pedicles, and scissors were inserted outside of the hysteroscopic sheath to cut the myoma pedicle). More recently the techniques have been refined to use scissors inserted through the operating sheath of the hysteroscope or use the resectoscope to shave sessile myomas to the level of or just below the endometrial surface (Fig. 26.9).
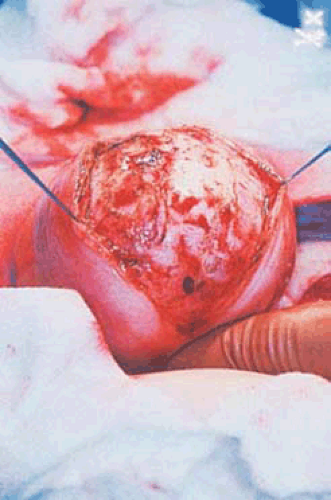 FIGURE 26.3 Intramural myomas must be removed by the abdominal route. In this case, the operator is using a laser to incise the uterus and to expose the capsule of the underlying intramural myoma. |
Hysteroscopic Scissors
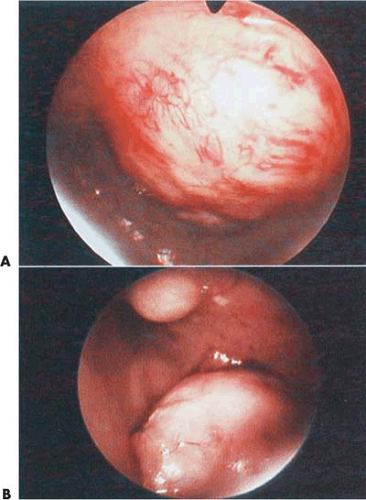
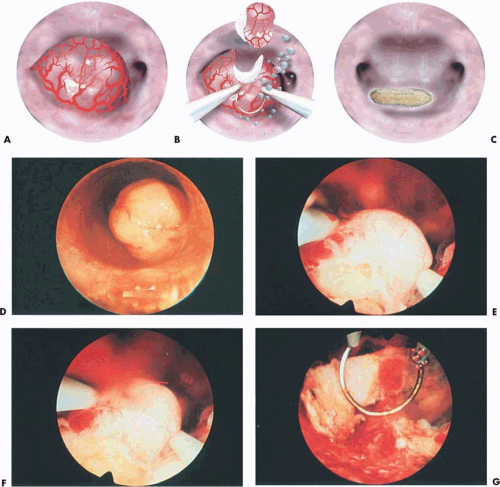
An operative hysteroscope, 7 to 8 mm outer diameter with an operating channel ranging from 2 to 3 mm, can be useful for submucous leiomyomas that are pedunculated and <3cm. The supporting pedicle of the leiomyoma should be well visualized and the manipulation simple. These leiomyomas can be transected easily, with or without previous coagulation of the pedicle. The transection is performed with semirigid or flexible scissors. The cervix is dilated and the tumor removed transcervically using grasping or toothed forceps. The uterine cavity is distended with low-viscosity fluids containing electrolytes, specifically sodium chloride (NaCl), or high-viscosity fluid (Hyskon) (Fig. 26.10).
Resectoscopic Myomectomy
The use of a gynecologic resectoscope has markedly improved and facilitated submucous myomectomy, particularly when the leiomyoma has a thick pedicle or is of the sessile type. In these situations, the leiomyoma is systematically shaved, using a cutting loop to reduce its size and then to remove the tumor fragments.
The resectoscope includes a straight-forward telescope (0 degrees) or a slightly fore-oblique 12- to 30-degree telescope with an outer diameter of 3 to 4 mm. This telescope can be encased in a system of concentric sheaths of 8 to 9 mm outer diameter. The instrument has a built-in system that provides motion by means of a spring and moves the distal cutting loop forward and backward to shave and resect lesions by electrical cutting. The outer sheath provides an outflow by small distal perforations that permit the fluid to exit once the uterine cavity is distended, while the inner sheath provides the inflow, which enters the uterine cavity directly for continuous irrigation. The outer sheath has a short distal insulated beak to protect against contact of the activated electrode with the outer metallic sheath. These two concentric sheaths are fitted to permit the introduction of an optical system and bridge for manipulation of various
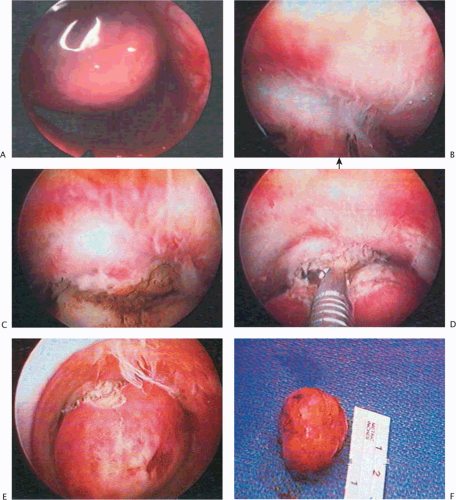
electrodes. To facilitate introduction, an obturator is available that fits the two assembled concentric sheaths. The flushing (constant in-and-out flow) resectoscope is inserted into the uterine cavity after the proper loop electrode has been selected and installed onto the sheath (Fig. 26.11A–C). The in-and-out trigger mechanism has likewise been tested prior to insertion into the uterus. Cutting current on the electrosurgical unit (ESU) is set at approximately 100 watts (W), and the coagulating current at 40 to 50 W. The myoma is located, the resectoscopic loop is advanced to the distal extremity of the myoma, and light contact with the surface of the myoma is made. Next, the electric current is activated by depressing the foot pedal, which is connected to the ESU. The electrode sinks into the myoma and is drawn toward the operator, shaving off a strip of myomatous tissue that floats in the medium. The procedure is repeated over and over until the base of the myoma is level with the surrounding endometrium (Fig. 26.11D–G). The fragments of tissue can be removed by withdrawal of the resectoscope from the outer sheath, allowing the “chips” to flow out of the cavity. Alternatively, they can be removed using a suction cannula via the cervix or inserting a polyp forceps.
electrodes. To facilitate introduction, an obturator is available that fits the two assembled concentric sheaths. The flushing (constant in-and-out flow) resectoscope is inserted into the uterine cavity after the proper loop electrode has been selected and installed onto the sheath (Fig. 26.11A–C). The in-and-out trigger mechanism has likewise been tested prior to insertion into the uterus. Cutting current on the electrosurgical unit (ESU) is set at approximately 100 watts (W), and the coagulating current at 40 to 50 W. The myoma is located, the resectoscopic loop is advanced to the distal extremity of the myoma, and light contact with the surface of the myoma is made. Next, the electric current is activated by depressing the foot pedal, which is connected to the ESU. The electrode sinks into the myoma and is drawn toward the operator, shaving off a strip of myomatous tissue that floats in the medium. The procedure is repeated over and over until the base of the myoma is level with the surrounding endometrium (Fig. 26.11D–G). The fragments of tissue can be removed by withdrawal of the resectoscope from the outer sheath, allowing the “chips” to flow out of the cavity. Alternatively, they can be removed using a suction cannula via the cervix or inserting a polyp forceps.
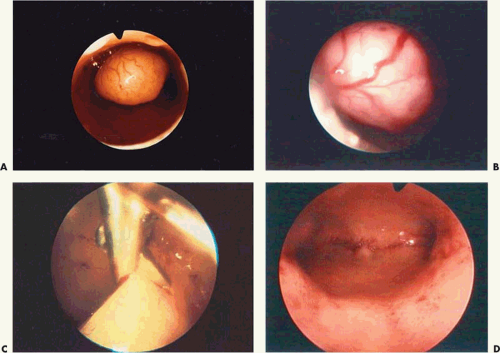 FIGURE 26.10 A: A 2-cm pedunculated myoma is clearly seen to emanate from the anterior uterine wall. B: Close-up view of the myoma shown in Figure 26.10A. The fragile surface vascularity is nicely demonstrated. C: Transection of a pedunculated myoma with scissors. The pedicle is rather narrow, and this is easily performed by operative hysteroscopy. D: The appearance of the uterine cavity 2 months after excision of the myoma. |
Two immediate problems exist intraoperatively when submucous myomas are excised by high-current density electrosurgical procedures (e.g., resectoscope shaving). The first difficulty is the problem of seeing clearly during the excision because of bubbles and debris being thrown up into the operative field. This difficulty can be ameliorated by using short bursts of electrical energy, 100 to 150 watts, rather than longer sustained activity. Bleeding from the myoma bed is the second difficulty. Even with rapid flushing, this bleeding can interfere with vision and make the surgical procedure riskier to perform. Hyskon has a definite advantage because the site of the bleeding is easier to identify. A ball electrode is best suited to coagulate open bleeding sites. Coagulation current at 50 to 60 W should be set on the ESU.
Loffer and others have observed that while resecting submucous myomas, particularly one with a shaved intramural component, the uterine wall contracts and pushes the intramural portion to the uterine cavity, permitting the complete resection of the myoma, if the intramural portion
does not involve the entire uterine wall. Litta et al. (2003) described a method for enucleation of the intramural component of a submucous myoma. The myomas ranged from 2 to 4 cm in 92.6% of cases, and the mean operating time was 27 minutes. If the myoma, however, did not extrude into the cavity, the authors felt it was hazardous to dig into the uterine wall with the resectoscope loop or for that matter any other device.
does not involve the entire uterine wall. Litta et al. (2003) described a method for enucleation of the intramural component of a submucous myoma. The myomas ranged from 2 to 4 cm in 92.6% of cases, and the mean operating time was 27 minutes. If the myoma, however, did not extrude into the cavity, the authors felt it was hazardous to dig into the uterine wall with the resectoscope loop or for that matter any other device.
Two important anatomic landmarks can assist the hysteroscopist in the removal of submucous leiomyomas: First, the consistency and texture of the fibrous tissue of the myoma are determined by gently touching the tissue with
the inactivated loop, permitting the pseudocapsule to be easily felt; and second, while the resection comes in contact with the juxtaposed myometrium, the fascicular myometrial tissue can be easily contrasted with the fibrous myomatous tissue. Also, the softness of the muscular tissue can be palpated with the inactivated loop, signaling the termination of the resection. Finally, the radius of the cutting loop electrode should be known and used at 2- to 3-mm depth when dissecting. Lin et. al (2001) have demonstrated a shortening of muscular fibers after removal of these myomas when no damage to myometrium has occurred, doubling the thickness at the site of myoma penetration.
the inactivated loop, permitting the pseudocapsule to be easily felt; and second, while the resection comes in contact with the juxtaposed myometrium, the fascicular myometrial tissue can be easily contrasted with the fibrous myomatous tissue. Also, the softness of the muscular tissue can be palpated with the inactivated loop, signaling the termination of the resection. Finally, the radius of the cutting loop electrode should be known and used at 2- to 3-mm depth when dissecting. Lin et. al (2001) have demonstrated a shortening of muscular fibers after removal of these myomas when no damage to myometrium has occurred, doubling the thickness at the site of myoma penetration.
The bipolar resectoscope permits the use of electrolyte-containing fluids, avoiding the development of acute hyponatremia; nonetheless, delayed fluid overload may still occur in susceptible patients. Therefore, vigilant monitoring of patients who absorb >1 L of isotonic fluid should be instituted.
An immediate look at the bed of the myoma with a resectoscope is useful to ensure that no remaining tissue has been left behind and to secure complete hemostasis.
Other Surgical Techniques Using Energy Sources
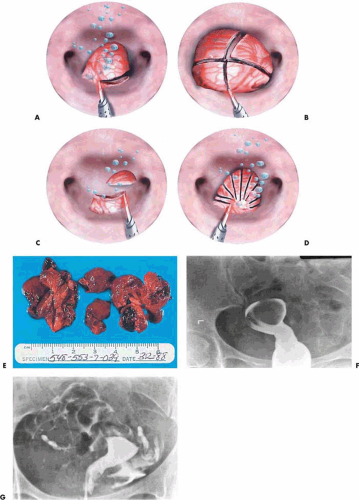
The neodymium–yttrium-aluminum-garnet (Nd-YAG) laser delivered by a quartz fiber through the operative channel of the operating hysteroscope may be used by means of several techniques (Fig. 26.12). First, either the pedicle of a pedunculated myoma or the sessile myoma may be cut off at the base by means of the laser alone (Fig. 26.13). The specimen is grasped with semirigid forceps and removed by pulling the entire sheath through the cervix. An alternative technique morcellates the myoma, whose component pieces are removed by ovum forceps or grasping forceps. A monopolar needle electrode (3 mm) may similarly be used via an isolated multichannel sheath to cut the myoma off its base followed by removal of the intact myoma via sponge forceps. The power required for this procedure is 60 to 100 W cutting current. Finally, the laser may be used to coagulate the base of the myoma, after which the flexible scissors are inserted through the hysteroscopic sheath to cut off the myoma. Actually, with the isolated channel operating sheath, the laser fiber and scissors may be simultaneously inserted into the uterus. The laser fiber may then be removed so that the coagulated edge of the myoma may be grasped with semirigid forceps and held on tension as the scissors cut through the tissue. The myoma may then be subdivided to facilitate removal. For myomas 2 cm or less in diameter, either the laser fiber or electrosurgical 3-mm ball may be used (60 to 100 W laser; 100 to 150 W electrosurgical) to ablate the myoma (Fig. 26.14A). The technique first coagulates the surface vessels with the defocused laser fiber or the ball electrode held just above the tissue. Next, the fiber or electrode is dragged repetitiously over the myoma until it is flattened. The only disadvantage with this method is the time expended to reduce the myoma. Another possible disadvantage is the lack of a tissue specimen for pathologic evaluation. For vascular lesions a long, flexible, 22- to 25-gauge needle may be inserted through the operative sheath and 1:100 Pitressin injected directly into the lesion before the cutting is initiated.
The Versapoint bipolar coagulating device is an excellent alternative to the Nd-YAG laser as a vaporizing-coagulating tool. As with the laser fiber or monopolar needle electrode, 2-cm submucous myomas may be simply ablated. Goldfarb (1999) presented 10-year follow-up data on combining endometrial ablative procedures with myoma ablation as well as stand-alone myoma ablation operations. Eighty-eight combination and 28 myoma-only ablations were followed up to 10 years. Eleven of the patients required a repeat surgical procedure. Of 52 women who had ablation of the endometrium only, 38% required repeat surgery. A ridged roller-barrel electrode, which develops very high power densities (current density), may be used for vaporization of small submucous myomas (Fig. 26.14B).
Alternatively, the myoma may be jabbed multiple times with the laser fiber, creating massive internal coagulation injury (myolysis). Deprived of its blood supply, the myoma essentially shrinks and dies on the vine. The technique can be used with 3-mm bipolar needles: The needles are thrust into the myoma 20 to 30 times, and bipolar electrical current is passed through the needles, necrosing the tissue between them (Fig. 26.15). Fifty watts of bipolar coagulation or 100 W of bipolar cutting current is exceedingly safe and very effective. The risk of adhesion formation is also very low with both the laser and bipolar myolysis.
Pedunculated myomas usually can be manipulated into a position to allow clear identification of the pedicle (Fig. 26.16). Vasopressin (Pitressin) 1:100 injection or Nd-YAG or electrosurgical coagulation is an alternative prior to cutting the pedicle. This step will usually eliminate pesky bleeding and relieve the surgeon of the necessity for inserting a balloon into the uterus. Postoperatively, the patient should be placed on antibiotics, since these lesions are usually
inflamed. Premarin (2.5-mg) may be administered daily to stimulate endometrial growth and epithelialization of the operative site. It is worthwhile to perform an office hysteroscopy to examine the cavity 6 to 8 weeks postoperatively. In some patients the excised myoma will regrow, and some patients will require further surgery. Of 26 patients operated on by Neuwirth, 9 required further surgery and 7 underwent hysterectomy. If conception is the goal of the patient, this should be attempted within 6 to 8 weeks postoperatively, as tumor regrowth is not predictable (Figs. 26.17 and 26.18).
inflamed. Premarin (2.5-mg) may be administered daily to stimulate endometrial growth and epithelialization of the operative site. It is worthwhile to perform an office hysteroscopy to examine the cavity 6 to 8 weeks postoperatively. In some patients the excised myoma will regrow, and some patients will require further surgery. Of 26 patients operated on by Neuwirth, 9 required further surgery and 7 underwent hysterectomy. If conception is the goal of the patient, this should be attempted within 6 to 8 weeks postoperatively, as tumor regrowth is not predictable (Figs. 26.17 and 26.18).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree