Shiga Toxin-Associated Hemolytic Uremic Syndrome
Mechanisms. Stx-HUS, the most frequent form of TMA, may follow infection by certain strains of
E. coli or Shigella dysenteriae, which produce powerful exotoxins (Stx).
3,
19 The term Shiga toxin was initially used to describe the exotoxin produced by
S. dysenteriae type 1. Then, some strains of
E. coli (mostly the serotype 0157:H7, but also other serotypes
such as O111:H8, O103:H2, O123, O26, and the O104:H4 strain isolated in the recent German outbreak)
20,
21 isolated from human cases with diarrhea were found to produce a toxin similar to the one of
S. dysenteriae . After food contaminated by Stx-producing
E. coli or
S. dysenteriae is ingested, the toxin is released into the gut and may cause watery or, most often, bloody diarrhea because of a direct effect on the intestinal mucosa. Stx-producing
E. coli closely adhere to the epithelial cells of the gastrointestinal mucosa causing the destruction of the brush border villi.
22 Stxs are picked up by polarized gastrointestinal cells via transcellular pathways and translocate into the circulation,
23 probably facilitated by the transmigration of neutrophils,
24 which increase paracellular permeability. Circulating human blood cells, such as erythrocytes,
25 platelets,
26,
27 and monocytes,
28 express Stx receptors on their surface and have been suggested to serve as Stx carriers from the intestine to the kidney and other target organs.
The disease is caused by two distinct exotoxins, Stx-1 and Stx-2, which are almost identical to the toxin produced by
S. dysenteriae type 1.
29 Both Stx-1 and Stx-2 are 70-kDa AB5 holotoxins comprising a single A subunit of 32 kDa and five 7.7-kDa B subunits. Interestingly, an AB5 toxin comprising a single 35-kDa A subunit and a pentamer of 13-kDa B subunits have been isolated from a highly virulent
E. coli strain (O113:H21) responsible for an outbreak of HUS, which may represent the prototype of a new class of toxins, accounting for HUS associated with strains of
E. coli that do not produce Stxs.
30 Despite their similar sequences, Stx-1 and Stx-2 cause different degrees and types of tissue damage, as documented by the higher pathogenicity of strains of
E. coli that produce only Stx-2 than of those that produce Stx-1 alone.
31,
32,
33 In a study in children who became infected by Stx
E. coli ,
E. coli strains producing Stx-2 were most commonly associated with HUS, whereas most strains isolated from children with diarrhea alone or from those who remained asymptomatic only produced Stx-1.
34 This is also true in mice and in baboons.
35Stx-1 and Stx-2 bind to different epitopes on the Gb3 molecules and they also differ in binding affinity and kinetics.
36 Surface plasmon resonance analysis showed that Stx-1 easily binds to and detaches from Gb3, in contrast to Stx-2, which binds slowly but also dissociates very slowly, thus leaving time enough for the cell’s incorporation.
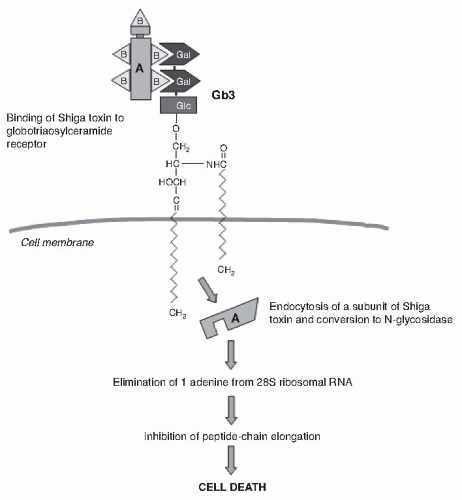
36 After binding to endothelial cell receptors, the toxin is internalized in the cytosol by endocytosis
37 within 2 hours
38 and inhibits protein synthesis within 30 minutes (
Fig. 55.9). The number of high-affinity receptors is a major determinant of susceptibility of cells to Stx.
39 Therefore, cell viability and protein synthesis of endothelial cells of the kidney were reduced by 50% upon exposure to 1 pM Stx, unlike endothelial cells of the umbilical vein that were viable up to greater than 1 nM exposure to the toxin. These findings are consistent with basal levels of Stx receptors 50 times higher in the renal endothelium than in the umbilical cord endothelium.
40 During internalization, the alpha subunit of the toxin dissociates from the beta subunits. Approximately 10% of the alpha subunit protein is removed in a trypsinlike process, resulting in a maximally active 27-kDa subunit enzyme. It is well established that this fragment is a direct inhibitor of protein synthesis and is responsible for the cytotoxic action of the toxin. Stx selectively inactivates 60S ribosomal subunits by removing one nucleotide in the 28S ribosomal RNA in a nucleotide-specific manner (
Fig. 55.9).
40For many years it was assumed that the only relevant biologic activity of Stx was the block of protein synthesis and destruction of endothelial cells. However, the treatment of endothelial cells with sublethal doses of Stx—exerting minimal influence on protein synthesis—leads to increased mRNA levels and protein expression of chemokines, such as interleukin (IL)-8 and monocyte chemoattractant protein-1 (MCP-1) and cell adhesion molecules, a process preceded by nuclear factor-kappa B (NF- kB) activation.
41 Adhesion molecules seem to play a critical role in mediating the binding of inflammatory cells to endothelium. Indeed, Stx-2 treatment enhanced the number of leukocytes adhering and migrating across a monolayer of human endothelial cells.
42 Moreover, preventing IL-8 and MCP-1 overexpression by adenovirus-mediated NF-KB blocking, inhibited adhesion and the transmigration of leukocytes.
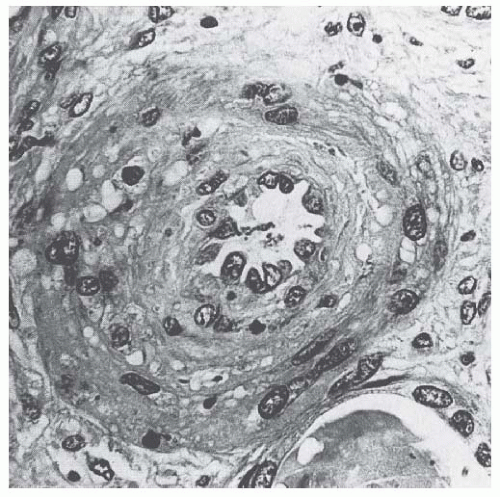
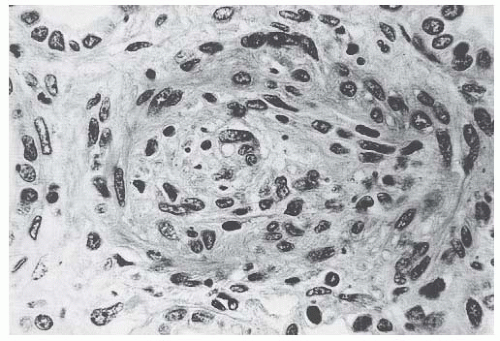
41Therefore, it can be inferred that Stx, by altering endothelial cell adhesion properties and metabolism, favor leukocyte-dependent inflammation and induce the loss of thromboresistance in endothelial cells, leading to microvascular thrombosis. Evidence for such a sequence of events has been obtained in experiments of whole blood flowing on human microvascular endothelial cells preexposed to Stx-1 at high shear stress.
43 In these circumstances, early platelet activation and adhesion occurs, followed by the formation of organized endothelial P-selectin and plateletendothelial cell adhesion molecule (PECAM)-1-dependent thrombi. This offers a likely pathophysiologic pathway for microvascular thrombosis in HUS.
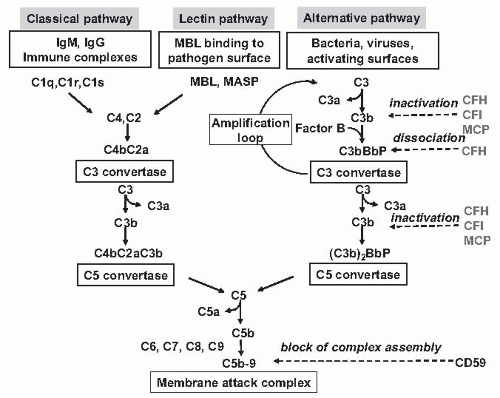
Evidence is also emerging that complement activation at the renal endothelial level may contribute to microangiopathic lesions in Stx-HUS. High plasma levels of complement activation products Bb and C5b-9 were recently measured in 17 children with Stx-HUS, indicating complement activation via an alternative pathway.
44 Another study reported that Stx2 binds to the plasma complement regulatory protein, factor H, and may activate complement in the fluid phase in vitro.
45 In a recent study, Stx-induced complement activation via P-selectin was identified as a key mechanism of microvascular thrombosis in Stx-HUS. Stx induced the expression of P-selectin on a cultured human microvascular endothelial cell surface, and P-selectin bound and activated C3 via the alternative pathway, leading to thrombus formation under flow.
46 In a murine model of HUS obtained by the coinjection of Stx2 and LPS and characterized by thrombocytopenia and renal dysfunction, the upregulation of glomerular endothelial P-selectin was associated with C3 and fibrin(ogen) deposits and platelet clumps. Treatment with anti-P-selectin Ab limited glomerular C3 accumulation. Factor B deficient mice after Stx2/LPS exhibited less thrombocytopenia and
were protected against glomerular abnormalities and renal function impairment, indicating the involvement of complement activation, via the alternative pathway, in the glomerular thrombotic process in HUS mice.
46Diagnosis. Diagnosis rests on the detection of
E. coli O157:H7 and other Stx-producing bacteria in sorbitol- MacConkey stool cultures. Unlike most other
E coli , serotype O157:H7 and other Stx-producing bacteria do not ferment sorbitol rapidly and thus form colorless colonies on sorbitol containing MacConkey agar (SMAC). Suspect colonies can be assayed for the O157 antigen with commercially available antiserum or latex agglutination kits. Newer protocols that use SMAC that contains cefixime tellurite, other selective culture media, immunomagnetic separation, and enzymelinked immunosorbent assays to detect O157 lipopolysaccharide or Shiga toxins can further enhance detection.
19 In regions where sorbitol-fermenting strains have been identi-fied,
19 the use of tests that identify Shiga toxins or the genes encoding them (by PCR) is helpful for diagnosis.
Over the last 2 decades
E. coli 0157:H7 and, although less frequently, other Stx-producing
E. coli strains, have been responsible for multiple outbreaks throughout the world, becoming a public health problem in both developed and developing countries.
19 Contaminated undercooked ground beef, meat patties, raw vegetables, fruit, milk, and recreational or drinking water have all been implicated in the transmission of
E. coli . A widespread outbreak associated with spinach in North America had dramatically higher than typical rates of both hospitalization (52%) and HUS (16%), due to the emergence of a new variant of the 0157:H7 serotype that has acquired several gene mutations that likely contributed to more severe disease.
47 From May through June 2011, a very large outbreak of HUS occurred in Germany and was caused by an unusual Shiga toxin- producing
E. coli (STEC) strain O104:H4 through the ingestion of contaminated sprouts.
48Secondary person-to-person contact is an important way of spread in institutional centers, particularly day care centers and nursing homes. Infected patients should be excluded from day care centers until two consecutive stool cultures are negative for Stx-producing E. coli in order to prevent further transmission. However, the most important preventive measure in child care centers is supervised hand washing.
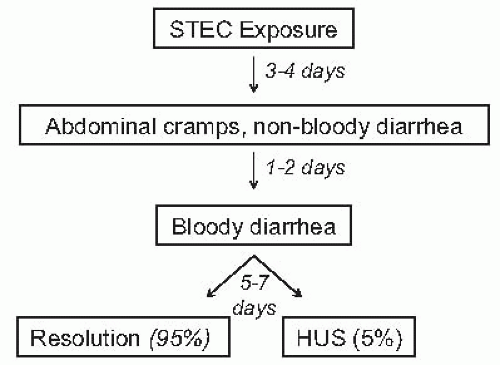
Clinical course. Following exposure to Stx
E. coli , 38% to 61% of individuals develop hemorrhagic colitis and 3% to 9% (in sporadic infections) to 20% (in epidemic forms) progress to overt HUS.
19,
49 Stx
E. coli hemorrhagic colitis not
complicated by HUS is self-limiting and is not associated with an increased long-term risk of high blood pressure or renal dysfunction, as shown by a 4-year follow-up study in 951 children who were exposed to a drinking water outbreak of
E. coli O157:H7.
50
Stx-HUS is characterized by prodromal diarrhea, followed by acute renal failure. The average interval between
E. coli exposure and illness is 3 days. Illness typically begins with abdominal cramps and nonbloody diarrhea; diarrhea may become hemorrhagic in 70% of cases, usually within 1 or 2 days.
51 Vomiting occurs in 30% to 60% of cases and fever in 30%. Leukocyte count is usually elevated, and a barium enema may demonstrate “thumb-printing,” suggestive of edema and submucosal hemorrhage, especially in the region of the ascending and transverse colon. HUS is usually diagnosed 6 to 10 days after the onset of diarrhea (
Fig. 55.10).
3 After infection, Stx
E. coli may be shed in the stools for several weeks after the symptoms are resolved, particularly in children < 5 years of age.
3Bloody diarrhea, fever, vomiting, elevated leukocyte count, extremes of age, and female sex, as well as the use of antimotility agents,
52 have been associated with an increased risk of HUS following an
E. coli infection.
19 Stx-HUS is not a benign disease. Of patients who develop HUS, 70% require red blood cell transfusions, 50% need dialysis, and 25% have neurologic involvement, including stroke, seizure, and coma.
19,
53,
54 Although mortality for infants and young children in industrialized countries decreased when dialysis became available, as well as after the introduction of intensive care facilities, still 3% to 5% of patients die during the acute phase of Stx-HUS.
53 A meta-analysis of 49 published studies (3,476 patients, mean follow-up of 4.4 years) describing the long-term prognoses of patients who survived an episode of Stx-HUS, reported death or permanent ESRD in 12% of patients and GFR below 80 mL/min/1.73m
2 in 25%.
54 The severity of acute illness, particularly central nervous system symptoms, the need for initial dialysis, and microalbuminuria in the first 6 to 8 months were strongly associated with a worse long-term prognosis.
54,
55,
56Disease presentation and outcome were particularly unusual during the STEC O104:H4 German outbreak, which since May 1, 2011 had involved more than 4,000 people in Germany, of whom 800 had progressed to HUS and 50 had died in Germany and 15 in other countries by July 20.
48 The outbreak was foodborne in contaminated sprouts.
21 The chain of transmission appeared to have started in Egypt with fecal contamination of fenugreek seeds by either humans or farm animals. During sprout germination, bacteria multiplied and produced large amounts of toxin and were then diffused with food provided to restaurants and consumers. Almost 90% of affected patients were adults and, compared to previous enterohemorragic
E. coli (EHEC) epidemics, there was a higher prevalence of affected young and middle-aged women and an extremely high incidence of dialysis-dependent kidney failure (20% versus 6%) and death (6% versus 1%), respectively.
20 The predominance of women among the case patients has been suggested to be explained by the food vehicle if women are more health conscious and thus more likely to eat sprouts.
20 Conversely, severe outcome was in part explained by a lack of previous immunity to this novel phenotype and, most likely, by the exceptional virulence of the strain.
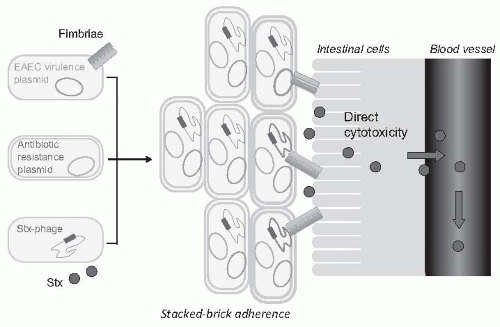
57 The involved O104:H4
E. coli shared 93% of the genomic sequence of enteroaggregative
E. coli (EAEC), microbes that form fimbriae that help sticking to the intestinal wall, but was also able to produce the same Shiga toxin of EHEC.
20,
58 Thus, this
E. coli is likely the result of an acquisition of Shiga toxin- encoding phage from a preexisting Shiga toxin-producing EHEC pathogen (
Fig. 55.11). Blending the two virulence factors would lead to stronger gut colonization and more toxin being released into the circulation. Moreover, although EHEC are found in the gastrointestinal tract of ruminants, EAEC have adapted to the human gut and appear to have their reservoir in humans.
59 This might explain why this strain has now acquired a host of new resistances to antibiotics most commonly used in human disease that are in large part mediated by extended-spectrum beta- lactamases (ESBL).
20Therapy. The typical Stx-associated HUS treatment for children is based on supportive management of anemia, renal failure, hypertension, and electrolyte and water imbalance. Intravenous isotonic volume expansion as soon as an
E. coli O157:H7 infection is suspected—that is, within the first 4 days of illness, even before culture results are available— may limit the severity of kidney dysfunction and the need for renal replacement therapy.
60 Bowel rest is important for the enterohemorrhagic colitis associated with Stx-HUS. Antimotility agents should be avoided because it may prolong the persistency of
E. coli in the intestinal lumen and therefore increase patient exposure to its toxin. The use of antibiotics should be restricted to the very limited number of patients presenting with bacteremia
61 because, in children with gastroenteritis, the risk of HUS increases by 17-fold.
62 A possible explanation is that antibiotic-induced injury to the bacterial membrane might favor the acute release of large amounts of preformed toxin. Alternatively, antibiotic therapy might give
E. coli O157:H7 a selective advantage if these organisms are not as readily eliminated from the bowel as are the normal intestinal flora. This might specifically apply to the O104:H4 strain, which has acquired a host of new resistances to antibiotics most commonly used in human diseases, such as cephalosporins, monobactams, fluoroquinolones, cotrimoxazole, tetracyclines, and aminoglycosides, which are in large part mediated by ESBL.
20,
63 Actually, ESBL-mediated resistances might offer to this strain a selective advantage over the normal intestinal flora upon exposure to one or more of the previous antimicrobials administered at the onset of gastrointestinal symptoms.
57 Moreover, several antimicrobial drugs, particularly the quinolones, trimethoprim, and furazolidone, are potent inducers of the expression of the Stx2 gene and may increase the level of toxin in the intestine. Although the possibility of a cause-and-effect relationship between antibiotic therapy and an increased risk of HUS has been challenged by a recent meta-analysis of 26 reports,
64 there is no reason to prescribe antibiotics because they do not improve the outcome of colitis, and bacteremia is only exceptionally found in Stx- associated HUS. However, when hemorrhagic colitis is caused by
Shigella dysentery type 1, early and empirical antibiotic treatment shortens the duration of diarrhea, decreases the incidence of complications, and reduces the risk of transmission by shortening the duration of bacterial shedding. Thus, in developing countries where
Shigella is the most frequent cause of hemorrhagic colitis, antibiotic therapy should be started early and even before the involved pathogen is identified. Whether early treatment with carbapenems or antimicrobials, such as fosfomycin, which are electively effective against ESBL-producing bacteria,
65 may help prevent progression from enterocolitis to HUS in patients with evidence or suspicion of gastrointestinal infection with
E. coli O104:H4 or other ESBL-producing strains may merit formal investigation.
Careful blood pressure control and renin-angiotensin system blockade may be particularly beneficial in the long term for those patients who suffer chronic renal disease after an episode of Stx-HUS. A study in 45 children with renal sequelae of HUS followed for 9 to 11 years documented that the early restriction of proteins and the use of ACE inhibitors may have a beneficial effect on the long-term renal outcome, as documented by a positive slope of 1/Cr values over time in treated patients.
66 In another study, an 8- to 15-year treatment with ACE inhibitors after severe Stx-HUS normalized blood pressure, reduced proteinuria, and improved GFR.
67An oral Shiga toxin-binding agent that may compete with endothelial and epithelial receptors for Shiga toxin in the gut (SYNSORB Pk) has been developed with the rationale of limiting target organs’ exposure to the toxin (
Table 55.2). However, a prospective, randomized, doubleblind, placebo-controlled clinical trial of 145 children with diarrhea-associated HUS failed to demonstrate any beneficial effect of treatment on disease outcome.
68 Among newer treatments for Stx-HUS, the development of Stx-neutralizing monoclonal antibodies, including dual antibodies against Stx 1 and 2 (SHIGATEC, NCT0152199) to be given at the time of gastrointestinal infection, is the most advanced.
69 Peptides impairing the ability of EHEC to survive under the acidic conditions of the gastric system could halt the disease process at even earlier stages by preventing bacterial intrusion into the gut.
70 Heparin and antithrombotic agents may increase the risk of bleeding and should be avoided.
Efficacy of specific treatments in adult patients is dif-ficult to evaluate because most information is derived by uncontrolled series that may also include atypical HUS cases. In particular, no prospective, randomized trials are available to definitely establish whether plasma infusion or exchange may offer some specific benefit as compared to supportive treatment alone. However, comparative analyses of two large series of patients treated
71 or not
72 with plasma suggest that plasma therapy may dramatically decrease the overall mortality of Stx
E. coli 0157:H7-associated HUS. These findings
lead us to consider plasma infusion or exchange suitable for adult patients, in particular in those with severe renal insuf- ficiency and central nervous system involvement.
Kidney transplants should be considered as an effective and safe treatment for those children who progress to ESRD. Indeed, recurrence rates range from 0% to 10%,
73,
74 and graft survival at 10 years is even better than in control children with other diseases.
75Evidence that uncontrolled complement activation may contribute to microangiopathic lesions of Stx-HUS
44,
45,
46 provided the background for complement inhibitor therapy in three children with severe EHEC-associated typical HUS who fully recovered with the anti-C5 monoclonal antibody eculizumab.
76 These encouraging results prompted nephrologists to use eculizumab therapy in HUS patients involved in the STEC O104:H4 outbreak in Germany (
Table 55.2). In the setting of a controlled multicenter clinical study (EudraCT, 2011-002691-17; Clinicaltrials.gov ID: NCT01410916) 148 patients with bloody diarrhea and/or evidence of STEC/EHEC infection, microangiopathic hemolysis, thrombocytopenia, renal insufficiency, and/or central nervous system complications or thrombosis received at least an 8-week eculizumab treatment. At the inclusion, 94 patients were on dialysis therapy, 22 required ventilatory support, and 129 were receiving plasma exchange or infusion. Outcome data were reported by Dr. Rolf Stahl from the Hamburg University Medical Center during the 43rd Annual Meeting of the American Society of Nephrology held in Denver in November 2011. No patient died. At 8 weeks, platelet count and serum creatinine levels normalized in 123 and 82 patients, respectively, and no patient had persistent seizures. Dialysis, ventilatory support, and plasma therapy were no longer required. Treatment was well tolerated in all patients and no case of meningococcal infection was reported. Comparative analyses versus 108 patients who had been treated with plasma exchange but without eculizumab showed remarkably larger and faster recovery in platelet count and kidney function in those receiving eculizumab therapy. Even better outcomes were observed in patients maintained on eculizumab therapy also after the completion of the originally planned 8-week treatment period. Altogether, the data clearly showed that complement inhibition by eculizumab therapy was lifesaving and almost fully prevented the risk of renal or neurologic sequelae in patients with severe diarrhea-associated HUS secondary to O104:H4
E. coli infection. Whether and to what extent these findings can be generalized to patients with more severe forms of typical HUS associated with gastrointestinal infections with other strains of Shiga toxin-producing
E. coli remains to be addressed.
Neuraminidase-Associated Hemolytic Uremic Syndrome
Mechanisms. This is a rare but potentially fatal disease that may complicate pneumonia, or less frequently, meningitis caused by
Streptococcus pneumoniae .
77 Neuraminidase produced by
S. pneumoniae cleaves N-acetylneuraminic acid from the glycoproteins on the cell membrane of erythrocytes, platelets, and glomerular cells.
78,
79 Removing the N-acetylneuraminic acid exposes the normally hidden Thomsen-Friedenreich antigen (T-antigen),
80 which can then react with anti-T immunoglobulin M (IgM) antibody naturally present in human plasma. This antigen-antibody reaction occurs more frequently in infants and children and causes polyagglutination of red blood cells in vitro. This is the reason why, unlike in other forms of HUS, in neuraminidase-associated HUS there is a positive Coombs test. T- anti-T interaction on red cells, platelets, and the endothelium was thought to explain the pathogenesis, whereas the pathogenic role of the anti-T cold antibody in vivo is uncertain.
81 T-antigen exposure on red cells is detected using the lectin hypogaea.
Clinical course and therapy. Patients, usually less than 2 years old, present with severe microangiopathic hemolytic anemia. The clinical picture is severe, with respiratory distress, neurologic involvement, and coma. The acute mortality is about 25%. The outcome is strongly dependent on the effectiveness of antibiotic therapy. In theory, plasma either infused or exchanged, is contraindicated, because adult plasma contains antibodies against the T-antigen that may accelerate polyagglutination and hemolysis.
80 Thus, patients should be treated only with antibiotics and washed red cells. In some cases, however, plasma therapy, occasionally in combination with steroids, has been associated with recovery.
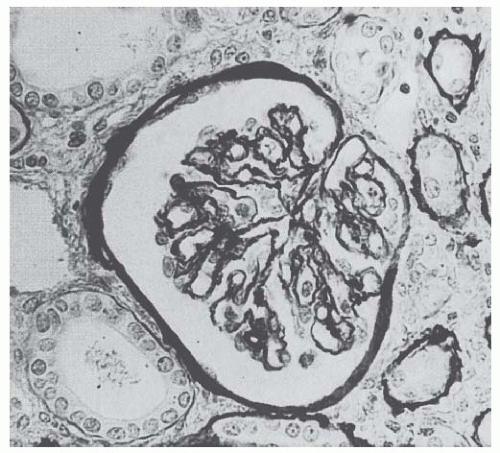
Atypical Hemolytic Uremic Syndrome. Atypical HUS (aHUS) includes a number of associations and presentations. It can occur sporadically or within families. Research in the last 10 years has linked aHUS to uncontrolled activation of the complement system (
Fig. 55.12 and
Table 55.1).
5
Familial atypical hemolytic uremic syndrome. Fewer than 20% of aHUS cases are familial. Reports date back to 1965, when Campbell and Carré described hemolytic anemia and azotemia in concordant monozygous twins.
82 Since then, familial HUS has been reported in children and, less frequently, in adults. Although some were in siblings, suggesting autosomal recessive transmission, others were across two to three generations, suggesting an autosomal dominant mode.
83,
84 The prognosis is poor (cumulative incidence of death or ESRD, 50% to 80%).
Sporadic atypical hemolytic uremic syndrome. Sporadic aHUS encompasses cases without a family history of the disease. Triggering conditions for sporadic aHUS
85 include HIV infection, anticancer drugs (e.g., mitomycin, cisplatin, bleomycin, gemcitabine), immunotherapeutic agents (e.g., cyclosporine, tacrolimus, OKT3, interferon, quinidine), antiplatelet agents (ticlopidine and clopidogrel), malignancies, transplantation, and pregnancy.
12,
86
De novo posttransplant HUS has been reported in patients receiving renal transplants or other organs, due to calcineurin inhibitors or humoral rejection. It occurs
in 5% to 10% of renal transplant patients who receive cyclosporine and in approximately 1% of those who are given tacrolimus.
87,
88,
89 TMA usually develops in the first weeks posttransplant when patients are treated with high doses of the immunosuppressant. Plasma exchange, combined with dose reduction or the withdrawal of calcineurin inhibitors, achieved remission in up to 80% of patients with de novo posttransplant HUS.
88In 10% to 15% of female patients, aHUS manifests during pregnancy or postpartum.
6,
85 aHUS may present at any time during pregnancy but mostly in the last trimester and about the time of delivery. It is sometimes difficult to distinguish it from preeclampsia. HELLP syndrome (HEmolytic anemia, elevated Liver enzymes, and Low Platelets) is a life- threatening disorder of the last trimester or parturition with severe thrombocytopenia, microangiopathic hemolytic anemia, renal failure, and liver involvement. These forms are always an indication for prompt delivery that is usually followed by complete remission.
85 Postpartum HUS manifests within 3 months of delivery in most cases. The outcome is usually poor.
About 50% of sporadic aHUS cases show no clear trigger (idiopathic HUS).


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access