Mitchell H. Rosner, Edgar V. Lerma, Sundararaman Swaminathan
Geriatric Nephrology
Aging is associated with a decline in kidney function, which can manifest as early as the fourth decade of life and accelerates between the fifth and sixth decades. These changes affect glomerular and tubular function, systemic hemodynamics, and body homeostasis. This chapter focuses on the management of the population older than 65 to 70 years.
Aging-Associated Structural Changes
Anatomic Changes
The human kidney reaches a maximum size of approximately 400 g (12 cm in length) in the fourth decade of life. A natural decline of approximately 10% in renal mass per decade then follows. This natural decline is associated with cortical thinning and decrease in the number of functional nephrons.
Glomerular Changes
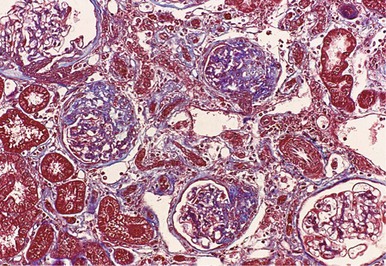
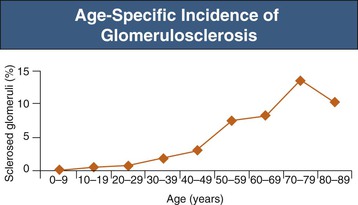
Structural glomerular changes with age include basement membrane thickening as well as development of focal and segmental or, rarely, global, glomerulosclerosis, which increases to 10% to 30%, and in some studies even exceeding 70% of glomeruli by the eighth decade (Figs. 67-1 and 67-2).1 Preserved glomeruli often show an increase in overall tuft cross-sectional area, consistent with glomerular hypertrophy.2 In aging mice and rats a strong relationship has been demonstrated between the glomerular hypertrophy and glomerulosclerosis, consistent with the hypothesis that glomerular hypertrophy may actually predispose to the development of glomerulosclerosis.3 Neither kidney function nor chronic kidney disease (CKD) risk factors can explain the strong association between age and glomerulosclerosis in healthy adults.4
Tubular and Interstitial Changes
Tubulointerstitial injury associated with aging is most pronounced in the outer medulla, with tubular dilation and atrophy, mononuclear cell infiltration, and interstitial fibrosis. Some tubules (especially in the distal tubule and collecting duct) may develop small diverticuli; it has been suggested that these diverticuli play a role in the development of upper urinary tract infections (UTIs; pyelonephritis) by harboring bacteria, thereby predisposing to recurrent infection.5
Vascular Changes
Arterioles often develop hyalinosis with aging. Thickening of the arterioles with an increase in the medial thickness/lumen diameter ratio is common with aging but is observed almost exclusively in hypertensive individuals.5 The arcuate arteries become more angulated and irregular with aging, and there is increased tortuosity and spiraling of the interlobar vessels. These changes occur independently of hypertension but are augmented in its presence. With aging, some afferent arterioles, particularly of juxtamedullary glomeruli, develop vascular shunts to the efferent arterioles, thereby bypassing glomeruli, leading to “aglomerular arterioles.”6
Aging-Associated Changes in Renal Function
Glomerular Filtration Rate
Inulin clearance studies document a progressive fall in glomerular filtration rate (GFR) after the age of 40 years, with a relatively greater decline in men (Fig. 67-3).7 However, the fall in GFR is not inevitable; in as many as one third of patients who remain normotensive, there is no decrease in creatinine clearance with age.5
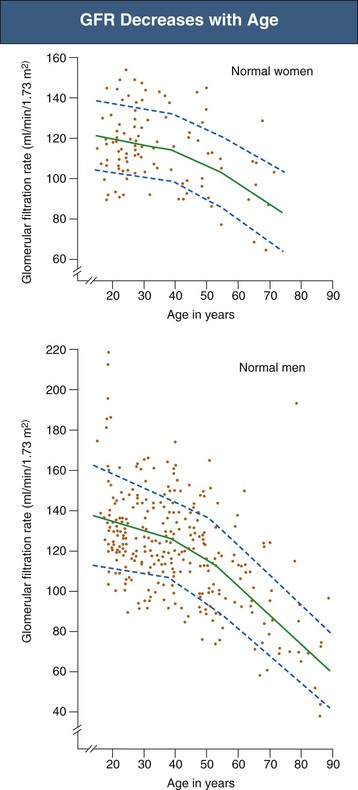
In addition to the decrease in GFR with aging, there may be a reduction in renal “reserve.” Whereas some studies suggest that aging humans show a normal increase in GFR after amino acid infusion, others have shown a marked reduction in increases in renal plasma flow (RPF) and GFR in response to concurrent infusion of amino acids and dopamine in healthy elderly individuals.8,9
Renal Plasma Flow
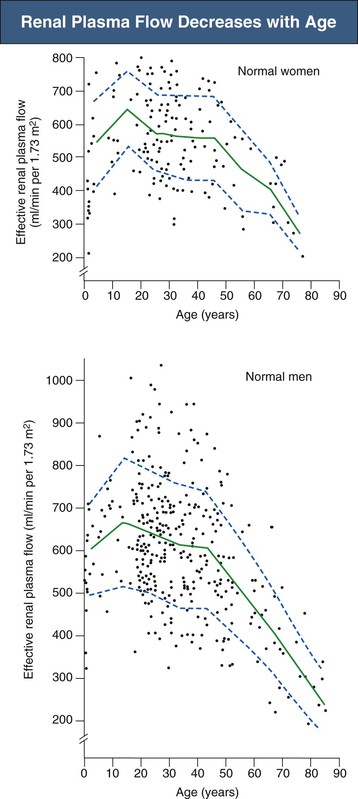
Renal plasma flow also decreases from a mean of 650 ml/min in the fourth decade to 290 ml/min by the ninth decade, with increasing renal vascular resistance (Fig. 67-4).5 The fall in RPF with age is greater in men than in women and in those who are hypertensive.10 Because RPF decreases relatively more than GFR, filtration fraction (defined as GFR/RPF) increases with age. Studies using a xenon washout technique demonstrated that there is a true reduction in renal blood flow when it is factored for renal mass.11 The decrease in renal blood flow especially involves the cortex, and blood flow to the medulla is relatively preserved.
Proteinuria
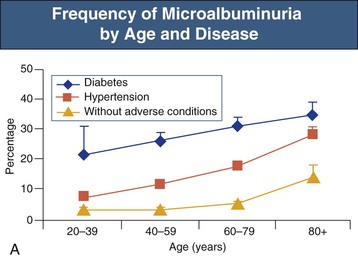
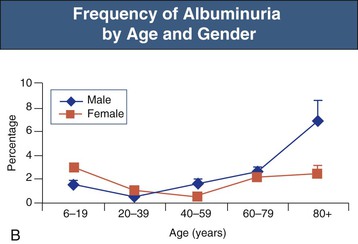
The prevalence of both microalbuminuria (urinary albumin levels of 30 to 300 mg/day) and albuminuria increases progressively after the age of 40 years. The increased prevalence is most marked in diabetic and hypertensive patients but is also observed in patients lacking these risk factors (Fig. 67-5).12
Assessment of Renal Function in the Elderly
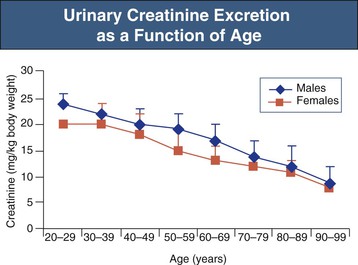
Serum creatinine is a less reliable indicator of renal function in the aging population. After the age of 60 years, there is a progressive decrease in urinary creatinine excretion, which largely reflects muscle mass decreases with aging (Fig. 67-6).13 For example, serum creatinine did not change between young patients and older patients despite a reduction in creatinine clearances from 140 ml/min/1.73 m2 at mean age 30 years to 97 at mean age 80 years.14
There is no consensus on the optimal approach to estimation of GFR in elderly people. Whereas the MDRD (Modification of Diet in Renal Disease) study equation and the Cockcroft-Gault formula for estimating GFR use age in their calculations (see Chapter 3), neither has been validated in people older than 70 years, and both underestimate true GFR in those older than 65 years with standard techniques such as isotope clearance. Although the MDRD equation may be more accurate than the Cockcroft-Gault formula,15 serum cystatin C, which is independent of muscle mass, may be superior to both and is an independent risk factor for mortality in the elderly.16 A new GFR-estimating equation was derived from 610 patients who were older than 70 years using iohexol clearance as the gold standard. This Berlin Initiative Study (BIS) equation worked particularly well in classifying patients with CKD stage 2 to 4 kidney function.17 More data are needed to determine which estimated GFR equation performs the best in the elderly patient.
Prevalence of Chronic Kidney Disease in the Elderly
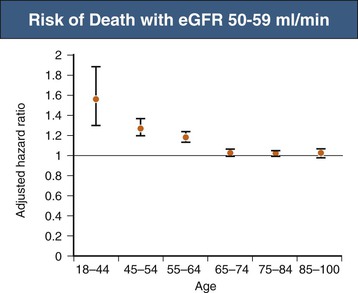
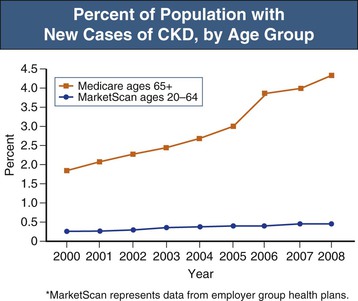
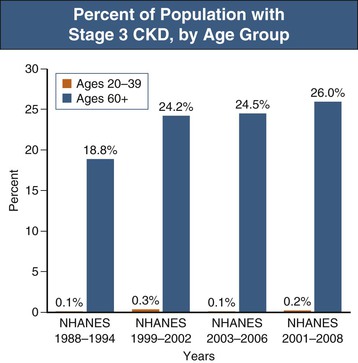
According to the United States Renal Data System’s 2010 and 2011 Annual Data Report as well as the National Health and Nutrition Examination Survey (NHANES) study, the incidence of CKD is increasing most rapidly in people aged 65 years and older, in whom the incidence of recognized CKD more than doubled between 2000 and 2008 (Figs. 67-7 and 67-8).18 CKD has been associated with an overall increase in all-cause cardiovascular mortality (see Chapters 79 and 82). Nevertheless, reductions of estimated GFR (eGFR) to 50 to 59 ml/min/1.73 m2 do not increase mortality risk among patients age 65 years or older compared with patients with eGFR of more than 60 ml/min (Fig. 67-9).19 These observations have led to the debate regarding whether the decrease in GFR that occurs with aging should really be considered unhealthy20 and whether the term “chronic kidney disease” in such cases should be replaced with “age-related reduced kidney function”.21
Risk Factors for Chronic Kidney Disease in the Elderly
The variability in the severity of aging-related renal disease in humans and experimental animals has suggested that there may be specific risk factors for its development. In experimental animal models, aging-related histologic changes vary according to the genetic strain, gender, body mass index, and diet. In animal models, aging-related changes can be retarded with protein or calorie restriction or blockade of the renin-angiotensin system (RAS).22 According to a multistage community-based survey, age, annual income, use of oral analgesics, metabolic syndrome, hyperuricemia, and hemoglobin were risk factors for CKD in both elderly and nonelderly patients.23 In elderly patients, previous medical history of diabetes mellitus, CKD, stroke, and analgesic use were positively correlated with CKD.23 In another prospective study, increased levels of physical activity correlated with a lower risk for rapid GFR decline (defined as loss greater than 3 mL/min/1.73 m2 per year in GFR, as estimated by longitudinal measurements of cystatin C levels) in a general population of older adults.24
Pathogenesis of Age-Related Chronic Kidney Disease
A variety of mechanisms have been proposed for aging-related renal changes (Box 67-1).
The kidney is the only known source of the anti-aging hormone Klotho. Mice with Klotho deficiency recapitulate features of systemic and renal aging. Klotho is synthesized by the distal nephron and is secreted as a circulating hormone.25 Klotho is a multipotent protein and serves as a cofactor for the phosphaturic hormone fibroblast growth factor 23 (FGF-23), regulates oxidative stress, and antagonizes transforming growth factor β (TGF-β) signaling. The mechanisms whereby Klotho regulates the aging process are being elucidated in animal models.
Additional mechanisms of renal aging may involve telomere shortening of chromosomal DNA, loss of mitochondria, and accelerated apoptosis. Increased numbers of apoptotic tubular and interstitial cells have been shown in the aging rat.26 This process may involve oxidative stress.27
Aging-associated renal disease may also be mediated by activation of the RAS, which may lower renal Klotho expression. In turn, in animal models, angiotensin type I recpetor (ATI) blockade upregulates Klotho,28 and in vivo Klotho gene delivery attenuated angiotensin II (Ang II)–induced renal damage.29 Treatment of rats with angiotensin-converting enzyme (ACE) inhibitors reduces aging-associated oxidative stress and preserves mitochondria in renal proximal tubules in association with upregulation of cellular anti-oxidant enzymes.30 Targeted disruption of the gene encoding for the ATIA receptor also results in marked prolongation of life span in mice.31 The longevity in these mice was associated with a decrease in cardiac and vascular injury possibly through attenuation of oxidative stress and overexpression of prosurvival genes (also in the kidney).
A loss of nephrons results in hyperfiltration with increased glomerular hydrostatic pressure and glomerular hypertrophy, which are known risk factors for glomerular scarring.32 Depending on the animal strain, glomerular hydrostatic pressures may be either elevated or normal with aging.22 It is thus likely that glomerular hypertension, when it occurs with a decrease in nephron mass, is a contributor to, rather than an initiator of, the aging-associated decline in renal function.
With aging, the elastic arteries dilate and stiffen, resulting in higher pulse wave velocity with increased pressure transmission to the microvasculature.33 Measures of large-artery stiffness are closely related to markers of renal microvascular injury, including albuminuria.34 Aging-associated intimal hyperplasia of the interlobular arteries and disease of the afferent arteriole may also alter renal autoregulation, resulting in injury to the glomeruli.35 Higher systemic pulse pressure, seen in aging individuals, has been associated with an accelerated decline in GFR.36
Endothelial function also declines with aging, particularly in men. It is associated with a progressive reduction in nitric oxide production by endothelial cells and is reflected clinically by a reduction in brachial artery reactivity.37 Loss of normal endothelial vasodilators may account for the increased renal vasoconstrictive response observed in aging rats to agents such as Ang II and endothelin 1 and may also contribute to the development of renal microvascular disease. Endothelial dysfunction can also inhibit renal angiogenesis, resulting in progressive capillary loss and ischemia. Indeed, mice lacking endothelial nitric oxide synthase show a marked acceleration of aging-associated renal disease.
Aging is also associated with more pronounced renal hypoxia, and ambient partial pressure of oxygen (Po2) is about 20 mm Hg lower in both the renal cortex and the medulla in otherwise healthy aged individuals compared with young patients.38 The reduced ability of the aged kidney to generate vasodilators may also contribute to renal hypoxia.39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree