A
B
Age
60 or older
50–59 and two of the following characteristics
Creatinine
>1.5 mg/dL
Hypertension
History of hypertension
Cause of donor death
Cerebrovascular death
While the ECD/SCD classification is simple and convenient for practitioners and patients, the dichotomous categorization fails to account for fact that organs may be misclassified by the rigid diagnostic criteria. It is now clear that some SCD kidneys may have functional characteristics of ECD organs (Fig. 9.1) and some ECD kidneys could be inadvertently discarded for fear of poor function and outcome. Baskin-Bay et al. noted nearly 11 % of deceased donor kidneys had worse posttransplant survival rates but were not classified as ECD [13].
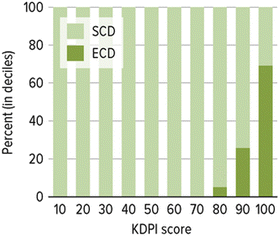

Fig. 9.1
Kidney donor profile index (KDPI) scores for ECD and SCD kidneys. KDPI scores mapped from kidney donor profile risk for 2011. The lower KDPI score represents kidneys with better functional and demographic characteristics. Higher scores are more likely to represent kidneys with less favorable characteristics. Reference Matas et al. [7]
To more accurately characterize donor kidneys, Rao et al. [14] have proposed a more granular index of risk, a continuous kidney donor risk index (KDRI). This index, based on data from the Scientific Registry of Transplant Recipients (SRTR), incorporates ten donor and four transplant characteristics that have been demonstrated to be independently and significantly associated with graft failure or death compared to a healthy 40-year-old donor. These factors include donor age, race, history of hypertension, history of diabetes, serum creatinine, cerebrovascular cause of death, height, weight, donation after cardiac death, hepatitis C virus status, human leukocyte antigen B and DR mismatch, cold ischemia time, and double or en bloc transplant. Analyzing nearly 70,000 deceased donors over a 10-year period, the authors noted the minimum KDRI was 0.5 and the maximal value was 4.2, although smaller and larger values were possible. It was noted the median KDRI was 1.05, which compares closely to the graft failure rate of the reference donor with a KDRI of 1.00. The KDRI is now remapped into a cumulative percentage scale from 0 to 100 using a kidney donor profile index (KDPI). This KDPI is easier to explain to patients as a donor with a KDPI of 80 % would have a higher expected graft failure rate than 80 % of kidneys recovered during the last year. See Fig. 9.1 for graphic representation.
Currently, UNOS has proposed a new kidney allocation policy which incorporates the KDRI classification. The public comment period closed in December of 2012 and a UNOS Board decision is expected in June of 2013 [15].
Benefits of ECD Organs
While understanding the variables used to classify ECD kidneys and the implications on graft outcome is important, applying this information for transplanting the appropriate organ in the best recipient is the ultimate goal. Ojo et al. provided the first evidence that transplanting “marginal” kidneys resulted in a tangible benefit. In his seminal study, waitlisted patients who received a kidney from older donors with either hypertension, diabetes, or prolonged cold ischemia had an average 5-year survival benefit compared to those patients who remained on the waiting list [16]. In 2005 Merion et al. further defined the benefits of transplantation using extended criteria donors [17].
Notable findings from the Merion study included a 17 % reduction in adjusted long-term mortality for recipients of ECD transplants compared to those who were waitlisted and may have eventually received a non-ECD organ. While there was a long-term improvement in mortality, the perioperative morality risk was 5.2-fold higher during the first 2 weeks posttransplant compared to the waitlisted group. This excess mortality risk eventually declined, and by 33 weeks the mortality risk equaled that in the waitlisted group. However, the substantial early risk resulted in a cumulative mortality that did not become equal in the two groups until 3.5 years posttransplant.
Management of ECD Organs
Understanding which organs are classified as ECD and the allocation system that distributes these organs is important but, ultimately, what is paramount is the appropriate management of these kidneys and choosing immunosuppression that will allow for both excellent short- and long-term graft survival.
Although it is convenient to address management of the recipient’s kidney transplant at the time of implantation, it is more appropriate to consider how the organ is preserved as this factor can have a crucial impact in the cascade of events during the first few days and weeks following transplantation. At the present time, there still remains no clear consensus as to whether static cold storage versus hypothermic machine perfusion (so-called pulsatile perfusion) is superior for organ preservation. While there are advantages to each method, improving short- and long-term outcomes remains the overall goal. Moers et al. helped to answer this important question in a well-done, large-scale, prospective randomized trial of 336 kidney donors in which each of the paired kidneys were randomized to either cold storage or machine perfusion [18]. After 1 year of follow-up, there was a significant reduction in delayed graft function, improved graft survival, and reduction of serum creatinine in the first 2 weeks following transplantation. In a follow-up to their initial study, the authors analyzed graft survival at 3 years [19]. Importantly, overall graft survival continued to be significantly better in the machine preservation group. Even more impressive was the improvement in graft survival from expanded donor kidneys in the machine preservation group compared to cold storage (86 % vs. 76 % with an adjusted hazard ratio of 0.38, P = 0.01). The authors then followed-up this study with an economic analysis that determined a net cost savings of over $8,000 for ECD using machine preservation [20].
A potential advantage of machine preservation is ex vivo perfusion of substrate to reduce ischemia-reperfusion injury and assessment of dynamic flow characteristics that can help make more informed decisions regarding acceptance of organ offers. However, neither histological assessment of donor kidneys nor machine perfusion characteristics have been reproducibly validated or have sufficient positive predictive value [21–23].
Irrespective of how the organ is preserved, cold ischemia time remains an important determinant of delayed graft function. Several studies have quantified the impact of duration of cold ischemia time in ECD transplants, and while there is a graded effect for increasing periods of ischemia, times greater than 10 h appear to have a significant effect [24, 25].
Impact of Donor Age
Kidneys from older donors are physiologically and immunologically different than kidneys from younger donors, and these changes make these kidneys more susceptible to damage and reduce the likelihood of meaningful recovery. Older kidneys have glomerular, interstitial, and vascular senescence [26, 27], reduced glomerular filtration rates, and overall worse renal function compared to younger recipients [27, 28]. A large study of older donors in Spain examined 3,365 transplants from donors older than 60 years, and at 1 year, their creatinine was significantly higher compared to younger donors, 2.1 versus 1.6 mg/dL (P < 0.0001) [29]. In addition to preexisting damage, older kidneys are at an increased risk for acute rejection [26, 27]. Fijter et al. demonstrated kidneys from donors >age 50 had a 1.53 times higher rate of rejection compared to younger donors [27].
One option available to allow a successful outcome with ECD organs is the transplantation of dual kidneys when one alone may not allow for satisfactory outcomes. This approach reduces organ discard rates while still achieving the goal of successful transplants in appropriate candidates. Tan et al. reported on their sizeable experience using dual kidney ECD transplants compared to standard donors. The 8-year actuarial graft survival was not different between the two groups, but waiting time was significantly reduced by 224 days (P < 0.01) [30].
Potential Implications of Using ECD Organs
A significant concern of transplant centers is that the utilization of extended criteria donor kidneys may result in poor outcomes that are publicly reported and used by the Center for Medicaid and Medicare Services (CMS) to determine if sanctioning and removal of public funding should occur. Schold et al. analyzed data from the SRTR from January 2007 to July 2009 and determined that 23 % of all adult kidney programs were considered to be low-performing centers as defined by lower than expected 1-year patient or graft survival [31]. These so-called low-performing centers had a reduction of over 22 transplants during the study period compared to an increase of nearly eight additional transplants at other centers. These low-performing centers had a decrease of all donor types, but ECD transplants declined by nearly five during this study period, suggesting that centers may view these kidneys as having unnecessary risk. This compared to an increase of nearly four additional ECD transplants at other centers. Therefore, understanding appropriate donor and recipient management strategies may help to reduce the risk associated with these kidneys.
Defining which patient group benefits (and just as importantly, who is harmed) from ECD kidney transplants is essential if transplant centers properly educate the patients and advise them to be listed for ECD transplants. Merion et al. analyzed over 109,000 waitlisted patients from 1995 to 2002 to determine which patients would benefit from ECD transplants [17]. After reviewing the results of nearly 8,000 transplants, they were able to demonstrate that the cumulative survival benefit of ECD compared to SCD transplants did not occur until 3.5 years, but the overall mortality risk was reduced by 17 % for patients who received an ECD transplant versus remaining on the waiting list. Significant benefit was specifically shown in the subgroup of patients under the following conditions: (1) median waiting times greater than 1,350 days: patients greater than age 40 and older, non-Hispanic, all races, and both sexes, and (2) median waiting times less than 1,350: only recipients with diabetes as the cause of ESRD demonstrated significant benefit. Figure 9.2 summarizes the proposed algorithm for ECD kidneys. Diabetics as a group experienced a 23 % reduction in long-term mortality when receiving an ECD kidney, likely due to the shorter waiting time and avoidance of dialysis complications. Patients less than age 40 did not benefit from ECD transplantation despite a shorter waiting time.
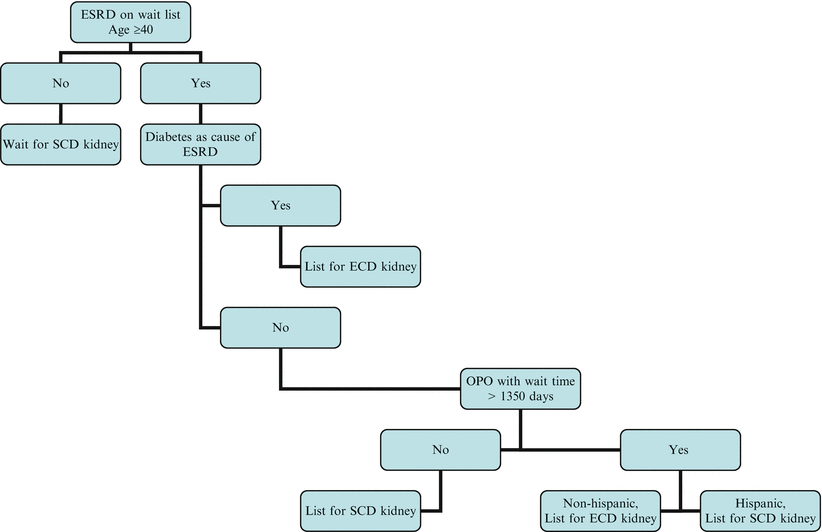

Fig. 9.2
Algorithm for listing patient for extended criteria donor kidney. Adapted from Merion et al. [17]
Traditionally, ECDs have referred to deceased donors, but with the ever expanding recipient waiting list, centers are now utilizing living donors that are older, obese, more often hypertensive, and with reduced renal function than previously. These living donors are now referred to as “medically complex” rather than extended criteria living donors. Regardless of the label, these living donor kidneys are at risk for delayed graft function, reduced long-term function, and less able to withstand challenges due to reduced nephron mass [32–34].
Although the data delineating which patients are the best candidates for ECD transplants has been available for over 7 years, there is still wide variability on how this is applied to candidates at the time of listing which has consequences for the patients’ overall waiting time and long-term survival. One large study demonstrated that only 50 % of patients that by Merion’s criteria [17] would benefit from an ECD kidney were listed for such an organ. Neither diabetic status nor length of the waitlist had any significant association with being listed for an ECD kidney [35]. Therefore, education of transplant professionals and patients is important to realize benefit from these organs.
Immunosuppressive Management
Immunosuppressive management that reduces acute rejection episodes and minimizes ischemic damage is the cornerstone of successful management of these fragile organs. The cardiovascular impact of renal dysfunction is not widely appreciated. A retrospective study of nearly 59,000 renal transplant recipients in the United States Renal Data System registry noted that a serum creatinine greater than 1.5 mg/dL at 1 year was strongly associated with cardiovascular death [36]. Therefore, minimizing renal dysfunction is important not only for graft survival but reducing patient mortality.
While there are multiple strategies to achieve these goals, the general concepts that most agree are useful include induction therapy to allow the delayed introduction of calcineurin inhibitors (CNI) and ultimately calcineurin elimination or minimization.
Delayed CNI introduction is possible with depleting antibody agents. A relatively large prospective trial using either rabbit anti-thymocyte globulin or alemtuzumab as the induction agent with delayed introduction of tacrolimus resulted in comparable rates of delayed graft function, acute rejections, and graft survival rates with a mean follow-up of 24 months in ECD kidneys compared to SCDs [37].
Recently, a novel immunosuppressive agent, belatacept has been used in renal transplantation as a costimulatory blocker that binds to CD80 and CD86 and has been demonstrated to allow for calcineurin inhibitor minimization. In the BENEFIT-EXT study, patients receiving an ECD kidney transplant were randomized to either belatacept or cyclosporine. At 3 years acute rejection rates were similar in each group, graft survival was not different at 82–80 %, but calculated GFR was 11 mL/min/1.73 m2 higher in the belatacept versus cyclosporine group. Moreover, this difference in GFR persisted and widened over time, from 8 ml/min at 1 year to 11 mL/min at 3 years [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







