Dyslipidemia
Goal
Initiate
Increase
Alternative
TG > 500 mg/dL with LDL < 100 mg/dL
TG < 500 mg/dL
TLC
TLC + niacin
Fibrate or statin
LDL 100–129 mg/dL
LDL < 100 mg/dL
TLC
TLC + low-dose statin
Ezetimibe or niacin
LDL > 100 mg/dL
LDL <100
TLC + low-dose statin
TLC + 50 % maximum-dose statin
Ezetimibe or niacin
TG > 200 mg/dL and non-HDL > 130 mg/dL
Non-HDL < 130 mg/dL
TLC + low-dose statin
TLC + 50 % maximum-dose statin
Ezetimibe or niacin
Antiplatelet Agents
The cardioprotective benefit of aspirin is well established. Aspirin is often started or continued in the immediate perioperative period for rheological reasons. This is especially true in patients where the allograft has small vessels or there was a need for vascular reconstruction at the time of surgery. Use of antiplatelet agents is a mainstay of therapy for prevention of cardiovascular events. In the FAVORIT study 38 % of patients greater than 6 years out from transplant were prescribed antiplatelet therapy as compared to 45 % of those less than 6 years since transplant. The increased use of antiplatelet agents in the more recent cohort may reflect an increased level of comfort in transplanting higher risk individuals. Patients are sometimes wary to use aspirin, even at cardioprotective doses, due to admonition from their transplant centers to avoid use of aspirin and nonsteroidal anti-inflammatory agents post-transplant.
Tobacco Use
It is estimated that between 13 and 33 % [65, 66] of kidney transplant recipients smoke. The risk of death is increased approximately 1.5–2.0-fold [67, 68] in recipients who smoke. Smoking cessation should be advised, ideally prior to transplant, but certainly post-transplant. In an analysis of USRDS data it was noted that smoking occurred in an average of 1.20 ± 0.88 years after transplant [68]. Of concern is how infrequently tobacco use is addressed in the primary care setting [69]. Patients are not always forthcoming about tobacco use, and in one assessment of smoking status with cotinine testing, 34 % of individuals who reported not smoking tested positive for cotinine [70]. As smoking is a chemical addiction, it is reasonable to incorporate methods utilizing nicotine replacement therapy with patches or gum during the cessation process. Pharmacologic therapy with varenicline (Chantix®) [71] is widely advertised and appears to be effective. No reports of use in renal transplant recipients have been published, though experience and personal communications indicate that it appears safe. For some, group therapy provides the best environment for successful smoking cessation. Regardless of the method or methods utilized, tobacco use should be addressed at each clinic visit and recommendations for smoking cessation discussed.
Diet and Exercise
After transplant several dietary restrictions are lifted. High phosphorus and high potassium foods, which were often restricted due to chronic kidney disease, are no longer restricted. Weight gain is typical post-transplant, often as a result of lack of dietary restrictions, improved gustatory satisfaction, and continued relatively sedentary lifestyle. Food may taste better as the often present metallic taste associated with renal failure resolves. Prednisone use is associated with an increase in appetite and many have attributed weight gain to the use of glucocorticoids. In a study by Painter [72], it was observed that the weight gains at 1 year post-transplant of recipients on prednisone were no different than that of those who had been withdrawn from prednisone. In the current era of immunosuppression, steroids are used in only 25–35 % of transplant recipients [3, 73] and it is therefore not as much of an issue.
The benefits of exercise and physical activity have been proven in the general population, as in Fig. 18.1 [74, 75]. A small study of prescribed exercise in renal transplant recipients demonstrated improved cardiorespiratory parameters, such as VO2max, but no difference in any of the measured biochemical parameters [76]. Of note in this study was a statistically significant decrease in the inflammatory cytokine IL-6 after 30 sessions of exercise training. A 5-year study by Zelle did show significant improvement in cardiovascular and all cause mortality in renal transplant recipients [77]. With reservation the conclusion was that there may be as much as a 40–50 % improvement in cardiovascular risk with increased physical activity in renal transplant recipients. Further support for increased exercise is noted in a study where exercise increased cardiorespiratory fitness [78]. Six months of exercise improved cardiac autonomic parameters as noted in VO2max, heart rate variability, and baroreflex sensitivity, factors that have been associated with decreased cardiovascular risk in other populations. Whether engagement in physical activity post-transplant confers the same cardiovascular benefits as it does pre-transplant remains controversial. A meta-analysis of 15 randomized controlled trials in solid organ transplant recipients failed to show benefit of exercise [79–81]. There was no demonstrable improvement in lipids, hypertension, post-transplant diabetes, or cardiovascular risk. However, all the trials were small, of relatively short duration, and had variable end points. Despite these controversies, exercise and physical activity should have only a positive impact on renal transplant recipients’ risk for heart disease.
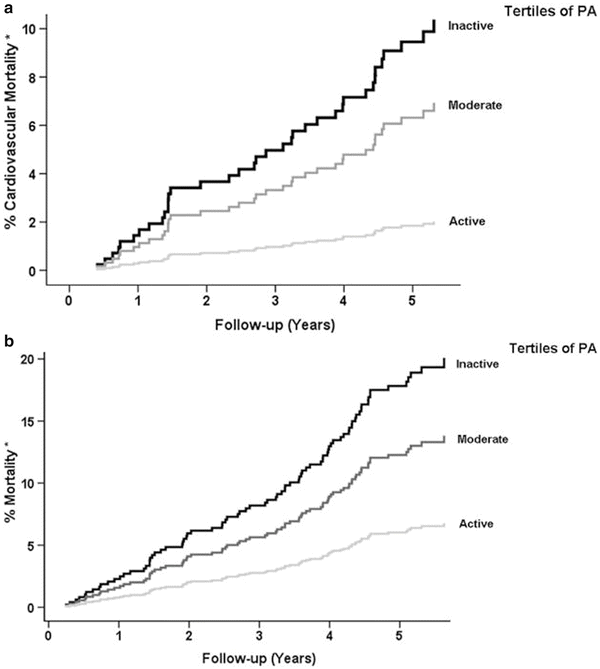

Fig. 18.1
(a) Kaplan–Meier curves of cardiovascular mortality according to gender-stratified tertiles of PA. *Adjusted for age (P < 0.001). (b) Kaplan–Meier curves of mortality according to gender-stratified tertiles of PA. *Adjusted for age (P < 0.001)
In summary, CVD is prevalent in the renal transplant population, less so than in the dialysis population, but more than the general population. Health care providers need to be more aggressive in addressing the risk factors contributing to CVD. Blood pressure should be adequately controlled to achieve a blood pressure ideally less than 130 mmHg systolic and less than 80 mmHg diastolic. Hyperlipidemia needs to be managed with the goal of achieving a total cholesterol of less than 200 mg/dL and an LDL of less than 100 mg/dL, with triglycerides of less than 150 mg/dL. Smoking cessation should be addressed at every visit for patients who are smoking. Weight control, healthy diet, and regular physical activity should be addressed, utilizing the expertise of a dietician and/or exercise physiologist if needed.
References
1.
Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97.PubMed
2.
Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LYC, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30.PubMed
3.
Matas AJ, Smith JM, Skeans MA, Lamb KE, Gustafson SK, Samana CJ, et al. OPTN/SRTR 2011 annual data report: kidney. Am J Transplant. 2013;13:11–46.PubMed
4.
Chico A, Tomás A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27(3):213–7.PubMed
5.
Delgado C, Johansen KL. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012;27(3):1152–7.PubMedCentralPubMed
6.
Rosas SE, Reese PP, Huan Y, Doria C, Cochetti PT, Doyle A. Pretransplant physical activity predicts all-cause mortality in kidney transplant recipients. Am J Nephrol. 2012;35(1):17–23.PubMedCentralPubMed
7.
Greenwood SA, Lindup H, Taylor K, Koufaki P, Rush R, Macdougall IC, et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant. 2012;27 Suppl 3:iii126–34.PubMed
8.
Friedman SE, Palac RT, Zlotnick DM, Chobanian MC, Costa SP. A call to action: variability in guidelines for cardiac evaluation before renal transplantation. Clin J Am Soc Nephrol. 2011;6(5):1185–91.PubMedCentralPubMed
9.
Nguyen KN, Patel AM, Weng FL. Ionizing radiation exposure among kidney transplant recipients due to medical imaging during the pretransplant evaluation. Clin J Am Soc Nephrol. 2013;8(5):833–9.PubMed
10.
de Mattos AM, Siedlecki A, Gaston RS, Perry GJ, Julian BA, Kew CE, et al. Systolic dysfunction portends increased mortality among those waiting for renal transplant. J Am Soc Nephrol. 2008;19(6):1191–6.PubMedCentralPubMed
11.
McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351(27):2795–804.PubMed
12.
Souza FLD, Bezerra KB, Sousa AR, Ferreira TCA, Oliveira MIG, Martins GP, et al. Estudo das alterações ecocardiográficas nos primeiros seis meses após o transplante renal. Arq Bras Cardiol. 2012;98(6):505–13.PubMed
13.
Sharma R, Pellerin D, Gaze DC, Gregson H, Streather CP, Collinson PO, et al. Dobutamine stress echocardiography and the resting but not exercise electrocardiograph predict severe coronary artery disease in renal transplant candidates. Nephrol Dial Transplant. 2005;20(10):2207–14.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






