Male gender
Age (>35 years)
Smoking history
Chemical exposure (benzenes or aromatic amines)
History of gross hematuria
History of other urologic disease
Irritative voiding symptoms
History of pelvic irradiation
Chronic urinary tract infection
Carcinogenic exposures: chemotherapy/alkylating agents
Chronic indwelling catheter or foreign body
Postoperative Hematuria
Immediate post-kidney transplant hematuria can be classified as surgical or pathological. Surgical hematuria is bloody urine that has resulted from or during the surgical procedure. Hematuria in this setting may result from bleeding at the site of the ureteral anastomosis or from Foley catheter trauma. In most cases surgical hematuria is self-limiting and requires no intervention. However, if the hematuria results in clinical instability, intervention may be required. A cystoscopic examination provides the diagnosis and if necessary the ability to fulgurate a bleeding surgical site.
Remote hematuria in the transplant recipient may be the result of kidney biopsy, rejection, or other pathologic entities. Immediate hematuria following a percutaneous renal biopsy is usually self-limiting. However, persistent hematuria following a kidney transplant biopsy may signify the formation of a pseudoaneurysm or an arterial venous malformation. These vascular injuries are best managed with angiography and embolization [48].
In the absence of surgical hematuria, infection and rejection merit consideration. Rejection may be associated with a decline of renal function. The diagnosis of rejection is determined with clinical judgment and/or renal biopsy. A urine culture is helpful to determine the presence of a bacterial or viral infection [49]. Urine cytology has historically played a role in the hematuria work-up. However, in the most recent AUA guidelines for hematuria work-up, urine cytology is not recommended as a necessary diagnostic test (Fig. 30.1). A formal hematuria work-up should be performed for transplant patients with risk factors for malignancy (Table 30.1).
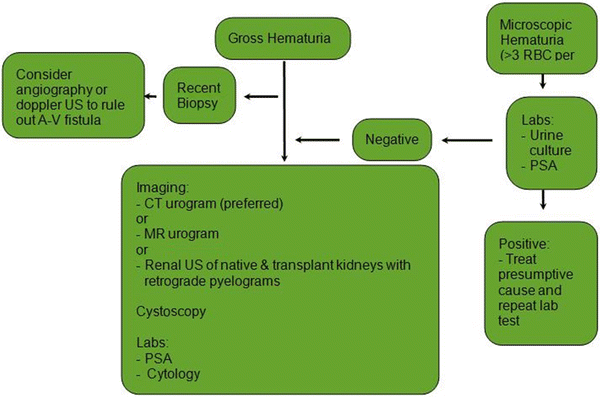

Fig. 30.1
Hematuria evaluation (Adapted from AUA Asymptomatic Microhematuria Guideline 2012)
Renal Cell Carcinoma
Renal cancer is the third most common urologic cancer. In 2008 there were about 54,000 new cases of kidney cancer. There are about 13,000 deaths per year secondary to kidney cancer [50]. Patients with renal failure, especially those with acquired cystic renal disease (ACKD), are at greater risk of developing renal cancer [51]. The number of people developing renal failure and requiring a kidney transplant increases each year [50]. With the increasing population of renal failure patients and the elevated risk of developing renal cancer in this patient population, one would project that renal cancer will be a growing problem. Furthermore, with the increased number of kidney transplants [52] and the associated survival benefit, there will likely be an increase in the rate of kidney cancer seen in this select population.
Preoperative Diagnosis of a Renal Mass
A suspicious renal mass can be detected as an incidental finding or as the result of a screening process. Many pre-transplant patients undergo abdominal imaging for a variety of reasons. The imaging studies frequently reveal an abnormal mass that is suspicious for renal cancer. The most definitive studies to characterize a renal mass are as follows: CT scan with and without IV contrast or MRI with and without IV contrast. In the pre-dialysis renal failure patient, these studies are not possible because of the contraindication to administrating IV dye due to nephrotoxicity or nephrogenic systemic fibrosis, respectively [53–55]. In this patient population, a renal ultrasound with Doppler flow is the best alternative. In the setting of a suspicious mass, the clinical management should be individualized. If the patient has already undergone a successful transplant, a native nephrectomy should be performed. The native nephrectomy can be performed with laparoscopic or open techniques. In most cases, the native kidney is small and laparoscopic technique is the preferred approach. Fortunately, renal cell carcinoma does not seem to flourish while under immunosuppression and therefore the immunosuppression does not need to be reduced [56]. However, urothelial carcinoma, which may develop in the renal pelvis, ureter, or bladder, can proliferate more rapidly and may require immunosuppressive adjustment as well as aggressive removal of the entire urothelial surface and creation of a urinary diversion with a bowel segment [32].
If a small, suspicious renal mass occurs in a patient who is not on dialysis, the renal mass can be observed with serial radiographic studies. Removal of the kidney prior to the initiation of dialysis may result in dialysis. In most cases renal cell carcinoma is a slow-growing tumor and close observation does not greatly increase the risk of metastasis. In this patient population, a nephrectomy is preformed upon the initiation of dialysis.
In the clinical scenario of a newly diagnosed renal cancer, the question of when it is safe to transplant often arises. In our institution, it has been our practice to proceed with transplantation without delay if the pathology of the native kidney is low grade and low stage. These “fast-track” clearance patients have recently been reviewed. Ten patients were transplanted following a recent nephrectomy for a renal cancer. The mean interval from the diagnosis of kidney cancer to transplant was 5 months. There were no recurrences or development of metastatic disease at a mean of 29 months (manuscript in preparation). If the patient has high-grade or high-stage renal cell carcinoma, we will typically wait for 18 months to rule out the development of metastatic disease or local recurrence.
Renal Mass in the Allograft
There are three clinical settings in which an allograft is identified with renal cancer. The most common scenario is when a renal mass develops in the allograft sometime following the renal transplant. The treatment options are allograft nephrectomy or a nephron-sparing procedure such as ablation or a partial nephrectomy. Partial nephrectomy remains a technically challenging procedure. Typically in a partial nephrectomy procedure, the renal hilum is occluded with vascular clamps. This allows the renal cancer to be excised in a bloodless fashion. In the transplanted kidney, access to the renal hilum is often impossible due to extensive scaring and adhesions. Partial nephrectomy procedures in allografts have been reported but are technically challenging [57, 58]. Renal tumor ablation with either radiofrequency or cryosurgery has been reported; however, the long-term outcomes of these techniques are pending [57]. The allograft nephrectomy should be reserved for large tumors or in patients in whom the graft has failed. The goal in managing the renal transplant patient with an allograft renal mass is twofold: adequate cancer control and preservation of a functioning transplant.
There are two other more controversial clinical scenarios in which an allograft renal mass or cancer occurs. The first is the discovery of a renal mass in a cadaveric kidney and the second is an incidental renal mass found on work-up of a live donor. Such allografts are typically rejected. However, with the ever-expanding gap between supply and demand of kidney allografts, there has been some initial successful experience with use of these organs that challenges this practice. In a cadaveric kidney, the renal mass is detected in the process of a back table bench preparation or on a CT scan. The partial nephrectomy is performed on a back table and the remaining unaffected kidney is used for transplantation [59, 60]. An incidental renal mass found on a live donor provides a challenge. This donor now must be faced with the issues of a diagnosis of a cancer and whether or not to proceed with the donation. Extensive informed consent is paramount for both the donor and recipient. Clearly, these are difficult issues and the use of an allograft with a renal mass probably should be reserved for high-risk recipients who may not survive an extended waiting period. For this patient population, the risks of a cancer recurrence are likely lower than the risk of dialysis.
Autosomal Dominant Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease (ADPKD) is a common cause of renal failure [61]. Nearly 10 % of the ESRD population is composed of ADPKD patients [62]. In addition to renal failure, many ADPKD patients have clinical problems directly related to the kidneys themselves. Renal colic, nephrolithiasis, and pyelonephritis are common. The mass effect of these kidneys may result in early satiety and shortness of breath. Moreover, the mass effect of some ADPKD kidneys is so great that there is no room in the patient to accommodate a renal allograft (Fig. 30.2). In these patients, bilateral nephrectomy may be required (Fig. 30.3). The bilateral nephrectomy can be performed in a staged fashion (before or after the transplant) or in a simultaneous fashion under the same anesthesia as the kidney transplant procedure. In pre-transplant patients, a staged pre-transplant nephrectomy will require a dialysis bridge until the patient is successfully transplanted. For many patients, a dialysis bridge is a major inconvenience. If a living donor is available, many patients would be eligible for a simultaneous bilateral nephrectomy and living donor transplant. This simultaneous approach has historically been avoided as it has been associated with risks and complications [63, 64]. Concomitant bilateral nephrectomy may be associated with longer operative times, increased blood loss, transfusions, and the potential of having the transplant cancelled if there is a surgical complication during the transplant. However, there is now a growing clinical experience with bilateral nephrectomy and simultaneous transplant with successful outcomes [65, 66]. The recent success may be associated with refinement in technique and improved surgical technology. The surgery is associated with large fluid shifts and hypotension and these may have detrimental effects on the transplant outcome if not managed appropriately. An experienced anesthesia team is therefore essential to the success of a simultaneous bilateral nephrectomy and renal transplant.
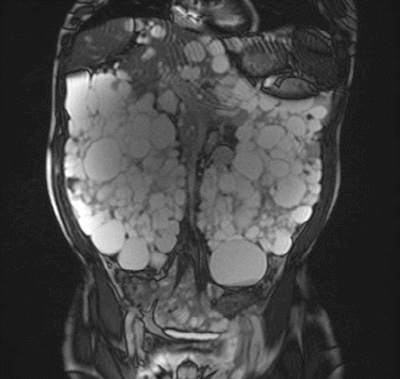
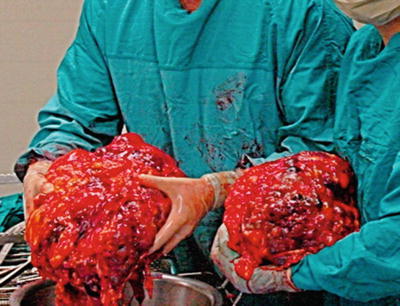

Fig. 30.2
MRI: polycystic kidneys

Fig. 30.3
Surgically removed polycystic kidneys
Renal Calculi
Renal calculi can occur in the native kidneys or the allograft. Incidental, asymptomatic stones in the native kidney do not require any intervention. However, if the burden in the native kidney is significant, it may present with pain or urinary tract infections. Pain in a native, atrophic kidney is rare; however, if the stone is obstructing a kidney that still produces urine, pain may develop. The surgical management of an obstructing stone is determined by the location and size of the stone. Proximal ureteral stones can be treated with extracorporeal shock wave lithotripsy (ESWL) or ureteroscopy and laser lithotripsy. Large native kidney stones associated with recurrent urinary tract infections (struvite stones) must be removed in order to successfully eliminate the infection. Removing the stone with endoscopic technique is an option; however, for stones >2 cm, endoscopic therapy may require multiple surgeries. In this particular scenario, one should consider a laparoscopic nephrectomy as an alternative approach.
Renal calculi may form de novo in the allograft or, alternatively, renal calculi may be transplanted and subsequently detected on radiographic studies. The incidence of allograft stones is about 0.4–1 % [67, 68]. The clinical management of asymptomatic calculi detected in a renal allograft should be individualized. For tiny asymptomatic stones, the best management may be observation. For stones that result in symptoms of obstruction, hematuria, or infection, urologic intervention is usually required. Allograft stones can be treated with ESWL [69, 70], ureteroscopy and laser lithotripsy [71], or percutaneous nephrolithotomy [68, 72, 73]. The goal of therapy is to eradicate the stone without injury to the transplant and/or native urinary tract. Surgical intervention in the transplanted kidney is challenging. ESWL is challenging as the pelvic bones shield the allograft from shock waves. Retrograde ureteroscopy is challenging because that transplant ureteral length is short and the neoureteral orifice is often difficult to access. The percutaneous approach is invasive and associated with risks including bleeding, vascular complications, and injury to overlying bowel. Nonetheless, the percutaneous approach is our preferred surgical approach in the setting of symptomatic allograft stones. In our experience, an interventional radiologist establishes the initial access which is then serially dilated over a short period of time to 16 Fr. A flexible cystoscope is then used in an antegrade fashion and the stone is obliterated with a holmium laser. If a ureteral stone is present, a flexible ureteroscope is used in an antegrade fashion to access the transplant ureter. From our experience with allograft nephrectomies, we have learned that the allograft is much more fragile than a native kidney. We therefore avoid active balloon dilation of an allograft for fear of bleeding or renal rupture.
Hydronephrosis
Hydronephrosis is a description indicating fullness in the renal pelvis and/or ureter that is frequently visualized on ultrasound examination of the transplanted kidney. Ultrasound of the allograft is a common diagnostic tool that is often used to evaluate elevated creatinine, blood flow, and renal anatomy during biopsy. A diagnosis of hydronephrosis of the transplant is not unusual and is not equivalent to a diagnosis of obstruction. A renal ultrasound is not a physiologic test and therefore the presence of obstruction cannot be established with ultrasound. In fact, an incidental diagnosis of hydronephrosis in the setting of a normal, stable creatinine probably does not require any further work-up. However, hydronephrosis in the setting of an elevated creatinine merits further investigation.
Vesicoureteral reflux, which is common in transplanted kidneys, may lead to the development of hydronephrosis. The simplest initial diagnostic step is to repeat the renal ultrasound with an empty bladder. An empty bladder can be established by Foley catheter or voiding immediately before the ultrasound. Dissipation of the hydronephrosis with these techniques rules out obstruction and further medical evaluation is required to determine the cause of the elevated creatinine.
Persistent hydronephrosis and elevated creatinine with an empty bladder suggest the possibility of a ureteral obstruction. Ureteral obstruction may result from ischemia or inflammation. Ureteral obstruction occurs in 2–10 % of cases making it one of the most common complications of renal transplant [74–76]. Seventy one percent of ureteral strictures occur within 3 months of the transplant [76, 77]. The majority occurs at the ureteral-vesical junction. Ureteral obstruction can be diagnosed with a MAG-3 lasix renogram. This is a physiologic study and provides information regarding uptake, excretion, and drainage. The patient should be well hydrated and the urinary bladder should be drained with a Foley catheter [78, 79]. The Foley catheter allows constant drainage and a more accurate assessment of the transplanted ureter which is very often difficult to interpret.
Ureteral obstruction secondary to inflammation or edema may be managed with a ureteral stent or a nephrostomy tube. These tubes can be removed when the inflammation or edema has resolved. The advantage of a nephrostomy tube is twofold. First, it relieves the obstruction and preserves renal function. Second, it also allows a diagnosis and anatomic evaluation of the level of obstruction. A nephrostogram is an excellent study to evaluate the anatomy of the obstruction. If the obstruction does not resolve within a reasonable time frame, the obstruction is likely secondary to stricture rather than edema.
Ureteral strictures often require surgical intervention. The surgical remedy depends on the length and location of the stricture. Ureteral reconstruction may require neoureteral anastomosis, pyelovesical anastomosis, or ureteroureteral (transplant ureter to native ureter) anastomosis. Reconstruction technique and strategy are individualized as the etiologies and location of the stricture may vary. For short ureteral strictures (2 cm) located at the ureteral vesical junction, an attempt at a minimally invasive repair is a reasonable first step. These strictures can be balloon dilated; however, the long-term success of balloon dilation ranges from 55 to 60 % [75, 80–84]. Given the high failure rate, modifications of the endoscopic balloon dilation have been implemented. Short ureteral strictures have been successfully incised with a holmium:YAG laser and balloon dilation [85].
References
1.
2.
3.
Ayub W, Fletcher S. End stage renal disease and erectile dysfunction: is there any hope? Nephrol Dial Transplant. 2000;15:1525–8.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






