Recipient blood group
Percent of population (%)
Donor blood group compatible with recipient
A
42
A, O
B
10
B, O
AB
4
A, B, AB, O
O
44
O
ABO blood group B frequencies are higher among individuals of African, Asian, and Central European descent. As an example, approximately 20 % of African Americans type as blood group B. In contrast, native Americans rarely express the blood group B allele. When ABO-incompatible blood is transfused into individuals a severe immune reaction can occur, including acute lung injury and fatal complement-mediated hemolysis. ABO-incompatible organs can be rejected immediately due to the presence of circulating preformed anti-A and/or anti-B antibodies. However in certain circumstances, transplantation across ABO disparate blood groups is possible. Individuals who are ABO blood group B or O may receive a kidney from an ABO A2 donor if their anti-A antibody titer is low (IgG ≤ 1:2) [1–4]. The A2 antigen is less reactive with anti-A isoagglutinin and is expressed in lower amounts on the surface of red blood cells and tissue cells [3]. Waiting times and outcomes for A2 kidneys transplanted into O or B recipients between 1995 and 2006 were examined using the United Network for Organ Sharing (UNOS) database [5]. There were 150,118 first kidney transplant recipients and among these 113 were O recipients of A2 kidneys and 125 were B recipients of A2 kidneys. These recipients had shorter waiting times than their counterparts who received ABO-compatible kidneys (A2 into O median wait time was 0.7 vs. 1.63 years and A2 into B median wait time was 0.74 vs. 1.9 years) [5]. In addition, there was no significant difference in graft or patient survival between the recipients of A2 compared with the ABO-compatible kidneys.
Several groups have developed protocols to transplant kidneys across major ABO barriers [6–11]. These protocols employ methods to reduce naturally occurring anti-A or anti-B antibodies. A variety of techniques and drugs have been used to achieve antibody reduction including plasmapheresis, intravenous immunoglobulin (IVIG), splenectomy, and rituximab [6–11]. These methods of antibody reduction have helped to expand the number of patients who may receive a kidney from a living donor. However, the growing participation of patients in paired donor exchanges may reduce the need for using ABO-incompatible donors.
Major Histocompatibility Complex
Major histocompatibility complex antigens (HLA) pose the next most important barrier to successful transplantation. HLA antigens are encoded by genes located on the short arm of the sixth human chromosome. This region, spanning an area of 3.6 mega base pairs, includes over 100 genes that are involved in the regulation of immunity. These genes are divided into three groups or classes. Class I and II genes encode for HLA molecules that are important in transplantation. Class III genes encode for other proteins related to the immune system, including heat shock proteins, complement factors, and cytokines. Class I HLA molecules consist of a heavy chain with three domains (alpha1, alpha2, alpha3) and an invariable light chain called beta-2 microglobulin (coded on chromosome 15). The alpha3 domain anchors the molecule into the cell, while the alpha1 and alpha2 domains form a peptide binding groove. Class II HLA molecules consist of two chains, an alpha and a beta chain (both coded on the sixth chromosome). Each chain has two domains, with the alpha1 and beta1 domains forming a peptide binding site.
The importance of HLA molecules hinges on their ability to present peptides to T cells. T cells, via their T cell receptor, are only capable of recognizing peptides (self or nonself) when these are presented in the peptide binding regions of HLA molecules [12–14]. T cells are continuously engaging HLA molecules to assess the nature of peptides. T cells will kill cells whose HLA molecules express nonself-peptides, such as viral peptides. This is how a person prevents viruses from spreading from cell to cell. Donor (allo) HLA molecules elicit a very strong immune response. Up to 15 % of all T cells are able to recognize nonself (allo)-HLA and initiate an effector response to a transplanted organ. It is because T cells are constantly surveying HLA molecules that HLA antigens are considered the major histocompatibility antigens.
HLA Class I genes include A, B, and C. HLA Class II genes include DP, DQ, and DR. HLA genes are co-dominantly expressed. Therefore, there are two HLA A molecules, two Bs, and so on. We inherit one number 6 chromosome from each of our parents. One set of HLA genes derived from one number 6 chromosome is called a haplotype. The probability that a sibling has inherited the same two haplotypes as a brother or sister who is in need of a transplant is 25 %, or 1 – 75 % (the probability that he/she inherited either one, 50 %, or inherited neither, 25 %). The probability that one of the several siblings is HLA identical (a two-haplotype match) is determined by the formula 1 − (0.75) n , where n is the number of siblings. Figure 2.1 illustrates the orientation of the HLA genes on chromosome 6. Figure 2.2 demonstrates the inheritance pattern of the HLA genes.
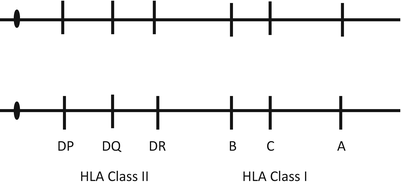


Fig. 2.1
Major histocompatibility complex/human leukocyte antigen on the short arm of chromosome 6 and their relative orientation on the chromosome

Fig. 2.2
The figure demonstrates the inheritance pattern for HLA genes. Each child is a one-haplotype match with its parent. Sibling 1 is a one-haplotype match to siblings 2 and 3, and a two-haplotype match with sibling 4. Sibling 2 is a one-haplotype match with siblings 1 and 4, and a 0 match with sibling 3. Sibling 3 is a one-haplotype match with siblings 1 and 4. Sibling 4 is a one-haplotype match with siblings 2 and 3. The probability that one of the several siblings is HLA identical (a two-haplotype match) is determined by the formula 1 − (0.75) n , where n is the number of siblings
HLA genes are highly polymorphic, with more than 1,980 unique alleles. These alleles are not randomly distributed, and certain alleles are more frequent than others. In fact, allele frequencies differ among different human populations [15]. Each HLA allele is unique in its ability to bind amino acids. The restricted nature of peptide binding favors having several different HLA molecules so many different peptides can be presented. If a person does not possess the ability to present peptides of a virus he/she will die because that virus will escape T cell-mediated elimination. The large number of HLA alleles (capable of presenting peptides) that exist in a population protects the population from extinction due to a specific viral infection. It is overwhelmingly likely that some members of the specific population will express HLA alleles that are capable of presenting peptides of all viruses to T cells. The fact that an individual has 12 distinct genes that code for HLA molecules makes it more likely that at least some peptides from a virus will bind in the peptide grooves of the HLA molecules to be presented to T cells, allowing T cells to kill virally infected cells.
HLA Class I is expressed on the surface of all nucleated cells while HLA Class II is expressed on antigen presenting cells (mononuclear phagocytes, B lymphocytes, dendritic cells) as well as some endothelial cells and thymus epithelium. Of note, HLA Class II is expressed on the endothelial cells of glomeruli and peritubular capillaries [16]. HLA of the transplanted organ can activate the recipient’s T cells via the direct and indirect pathways of T cell activation [17]. Recipient T cells residing in lymph nodes can be directly activated by donor passenger cells from the allograft migrating to local draining lymph nodes. The direct pathway is dominant early after transplantation. The indirect pathway is the classic pathway of T and B cell activation used by the immune system to combat microorganisms. Recipient antigen presenting cells process donor antigen first and then present donor peptides to recipient immune cells. This pathway is responsible for rejection episodes that occur later after transplant, after the passenger donor cells are no longer around to directly activate recipient immune cells.
Preformed Anti-HLA Antibodies
The presentation of donor HLA via the direct or indirect pathway can lead to the development of anti-donor HLA antibodies. Anti-HLA antibodies do not occur naturally (as do anti-ABO antibodies). There are three ways that an individual can be exposed to HLA antigens and subsequently develop anti-HLA antibodies. The first is via blood product transfusions. The second is via pregnancy and the third is via tissue transplantation. Preformed anti-HLA antibodies represent another major barrier to a successful transplant either by limiting the number of compatible donors or, worse, by causing early graft failure if transplantation occurs despite the presence of donor-specific anti-HLA antibodies. Donor-specific antibodies (DSAs), if present in high amounts, will cause immediate (hyperacute) graft loss and if present in small amounts will limit the survival of an allograft.
Influence of Mismatched HLA Antigens on Transplant Outcomes
Mismatched donor HLA antigens become a target of the immune response by a recipient. Unfortunately, not all recipients will have the opportunity to receive a 0-antigen-mismatched kidney. The outcomes of this type of transplant are superior as demonstrated by data tracked by the Scientific Registry of Transplant Recipients (SRTR) illustrated by Fig. 2.3 [18].
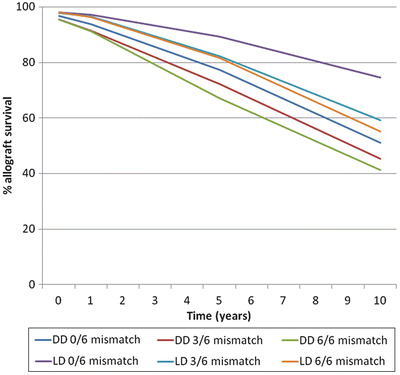

Fig. 2.3
Survival of deceased donor (DD inclusive of non-expanded criteria donor and expanded criteria donor) and living donor (LD) allograft kidneys according to their HLA mismatches with the recipient. Data for this figure was obtained from the OPTN/SRTR 2010 Annual Data Report (ADR) Table 5,10c, d as of October 1, 2010
The best opportunity for finding a minimally mismatched donor is among family members and specifically among siblings. However, for patients who do not have a living donor the national 0-antigen mismatch sharing program provides an opportunity to improve graft survival from a deceased donor. Patients who benefit the most from this program are those who possess anti-HLA antibodies and are therefore limited to a small pool of compatible donors. To qualify for this sharing program a patient must have a calculated panel reactive antibody (cPRA) (see below) of ≥20 % [18]. Not all patients will qualify for this program and even for those who do, if they possess a rare HLA phenotype, it is unlikely that a 0-antigen-mismatched donor will ever be identified. There is good evidence that long-term allograft survival is directly related to the number of HLA mismatches at the time of transplantation. A study of over 30,000 first deceased donor allograft recipients transplanted between 1984 and 1990 showed that 0-antigen mismatch kidneys had 1- and 5-year survivals of 84.3 % and 65.4 %, respectively. The 1- and 5-year survivals for 6-antigen-mismatched kidneys were 76.1 % and 52.3 %, respectively. There was also a stepwise decrease in survival with each mismatched HLA antigen [19]. Another study of nearly 136,000 recipients from 363 centers, transplanted between 1985 and 1994, reported 5-year allograft survivals of 69.9 % and 54.3 % for a 0- and 6-antigen mismatch, respectively [20]. This study also noted a persistent importance of HLA mismatching for recipients transplanted between 1995 and 2004, well after the introduction of superior immunosuppression, and also a stepwise decrease in 5-year allograft survival with an increasing HLA antigen mismatch [20]. Other studies have shown that greater levels of HLA mismatch are associated with higher rates of rejection [21, 22].
Pretransplant Assessment of Anti-HLA Antibody Status
The pretransplant assessment for anti-HLA antibodies consists of determining the breadth and strength of anti-HLA antibodies that are present and performing a crossmatch prior to transplant to be certain that there are no DSAs.
There are several reasons for determining if anti-HLA antibodies are present in a patient’s serum prior to transplant. First, it is known that patients with a high level of anti-HLA antibodies have a higher incidence of rejection [23, 24]. Most transplant programs will give stronger immunosuppression to patients who are sensitized (possess high levels of anti-HLA antibodies). Second, patients who have anti-HLA antibodies receive additional points in the allocation scheme and are prioritized for transplantation with a deceased donor [18]. Third, by determining to which specific HLA antigens a patient has antibodies, it is now possible to list those on the national computer (UNet, the computer program operated by the UNOS, the national organ procurement and transplantation network) as unacceptable antigens [18, 25–28]. Their policies and guidelines regarding the listing of unacceptable antigens can be found on their website. If listed in UNet as an unacceptable antigen for a specific patient, kidneys from donors with that antigen will not be offered to that patient. Moreover, the only way to obtain allocation points for being sensitized is to list unacceptable antigens in UNet (more later). Fourth, and finally, it is very useful for the physicians caring for a patient who is on the waiting list to know if a patient is sensitized. The more sensitized a patient, the longer it will take to find a compatible donor, as illustrated by SRTR data in Table 2.2 [18]. This is important information for both the patient and his/her physician.
Table 2.2
Time (years) to transplantation according to percentage of panel reactive antibodies (PRAs)
Peak PRA | ||||
|---|---|---|---|---|
Total patients | 0–9 % | 10–79 % | ≥80 % | |
10th percentile (TT) | 0.29 | 0.31 | 0.43 | 0.52 |
25th percentile (TT) | 0.94 | 0.93 | 1.32 | 1.97 |
50th percentile | 3.50 | 3.18 | 4.86 | Not enough patients to calculate |
The techniques for determining the presence of anti-HLA antibodies have evolved. The commonly used term to describe the breadth of antibodies is PRA. PRA stands for panel reactive antibody and is expressed as a percent. The techniques for the determination of an individual’s PRA are illustrated in Fig. 2.4.
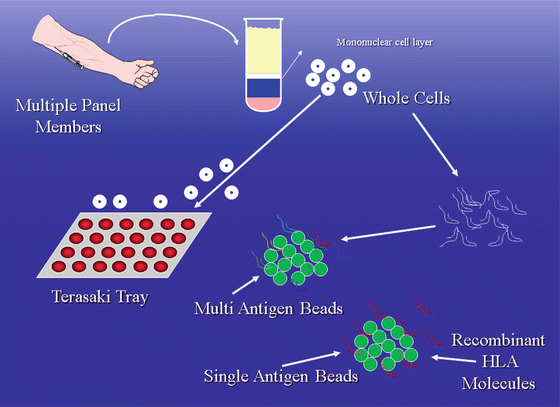

Fig. 2.4
Panel reactive antibodies. Two different techniques: cytotoxicity method and bead-based method. The cytotoxicity method involves the use of whole cells from a panel of donors who together possess as many known HLA antigens as possible. Each donor has his/her cells placed into one well of a tray and mixed with the recipient serum and complement. Cytotoxicity as evidenced by dead donor cells in a tray well represents reactivity of the recipient sera with the donor. The percent PRA is the number of “reactive” wells, or wells with dead cells, divided by the total number of wells. Example: 12 wells with dead cells and a total of 48 wells = PRA of 25 %. The current techniques are bead based. Polystyrene or latex beads are coated with HLA molecules that are obtained from either digested cells from multiple donors (multi-antigen beads) or recombinant techniques (single antigen beads). A mix of either Class I or II beads is chosen to represent as many HLA antigens as possible. The “flow PRA” uses multi-antigen beads, patient serum, fluorochrome-tagged anti-human immunoglobulin antibodies, and a flow cytometer to detect anti-HLA antibodies. In the assay, beads that have antibodies attached indicate that the patient possesses anti-HLA antibody to the HLA bound to the bead. The “reactive beads” emit photons from laser activation of fluorochromes on the antibodies. The PRA percent is calculated by counting the number of beads that have antibodies attached and dividing by the total number of beads present. For instance, if 30 % of the beads have antibodies attached the flow PRA is 30 %
The classical method for determining the PRA used a panel of individuals who together possessed as many of the known HLA antigens as possible [29]. Cells from these individuals were put on a tray, each well containing the cells of one individual. Serum from a patient was added to the tray to determine if antibodies were present, the readout being cytotoxicity in the presence of complement. The PRA percent was calculated by simply dividing the number of wells with dead cells by the total number of wells (e.g., 24 wells with dead cells and a total of 48 wells = PRA of 50 %). The cytotoxicity method is less sensitive than techniques that are currently used but the term PRA has endured. Current techniques are bead—instead of cell—based. Polystyrene or latex beads are coated with HLA molecules that are obtained from either digested cells (multi-antigen beads) or recombinant techniques (single antigen beads). The “flow PRA” uses multi-antigen beads, patient serum, fluorochrome-tagged anti-human immunoglobulin antibodies, and a flow cytometer to detect anti-HLA antibodies. Multi-antigen beads have either HLA Class I or Class II antigens attached [30, 31]. A mix of either Class I or II beads is chosen to represent as many HLA antigens as possible. The mix does not represent the frequency of HLA antigens in the population because this varies by demographic group. In the assay, beads that have antibodies attached (indicating that a patient has an anti-HLA antibody(ies) that recognizes the HLA antigen(s) that is (are) bound to that bead) emit photons from laser activation of fluorochromes on the antibodies. The PRA percent is calculated by counting the number of beads that have antibodies attached and dividing by the total number of beads present. If 10 % of the beads have antibodies attached the flow PRA is 10 %. Another technique using multi-antigen beads (quick screen) can be used to determine if a patient has anti-HLA antibodies, but the breadth of antibodies (PRA) is not calculated [32]. When either the flow PRA or quick screen is positive a single antigen bead assay is generally run to identify the specific antibodies causing the positive results. Single antigen bead assays use a Luminex platform that in addition to recognizing the presence of antibodies attached to beads can identify intrinsic color of the beads [33, 34]. Polystyrene beads with multiple (up to 1,000) different colors are used in these assays. The advantage of this platform is the ability to attach specific recombinant HLA molecules to beads having a unique color. For example, HLA B7 adherent beads will have a different color from HLA A2 adherent beads. Luminex assays can determine if antibodies are bound to the HLA A2 beads, to the HLA B7 beads, to both, or to multiple other beads with unique colors and unique single HLA antigen specificities.
For patients who are waitlisted on the national computer for a deceased donor a PRA is calculated (cPRA) by entering unacceptable antigens into UNet. The computer is programmed to calculate a cPRA based on the frequency of HLA Class I and Class II antigens expressed by 12,000 US organ donors [18, 35]. The higher the cPRA, the greater the number of points offered to a patient via the allocation scheme. A high cPRA can result from a small number of common HLA antigens (e.g., HLA A2) or from a large number of less common HLA antigens. It is the responsibility of each transplant program to enter unacceptable HLA antigens for each of their waiting patients into UNet. Most patients will have none. Some will have one or more and a few will have many unacceptable antigens. An unacceptable HLA antigen may be defined differently by different programs and currently there is no national standard. HLA antigens will be listed if they exceed a threshold based on the strength or amount of antibody measured. The measure of strength in the Luminex single antigen bead assay is mean fluorescence intensity (MFI). Increasing fluorescence intensity measured by photons emitted by specific beads bound with specific HLA molecules correlates with increasing amounts of antibody to that specific HLA antigen. Each program, in consultation with their tissue typing laboratory director, chooses an MFI threshold above which an HLA antigen will be listed as unacceptable. If the MFI threshold is low, more HLA antigens will be listed and if the threshold is high fewer will be listed. At present MFI thresholds chosen by programs range from as low as 1,000 to as high as 10,000. One distinct advantage of listing unacceptable HLA antigens in UNet is that when a donor kidney is offered to a patient it is highly likely that the final pretransplant crossmatch (see below) will be compatible. However, this is much more likely if the unacceptable MFI threshold is set low and much less likely if the MFI threshold is set high.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






