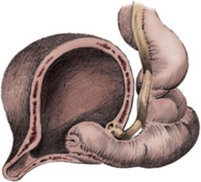
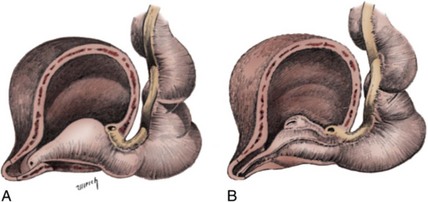
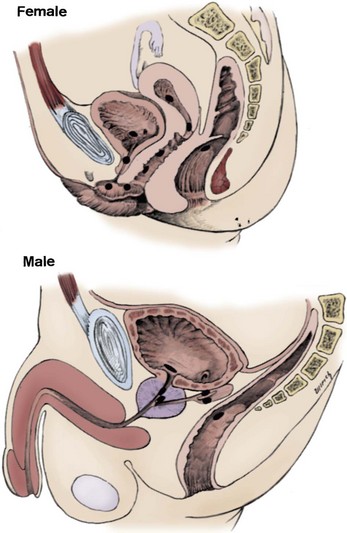
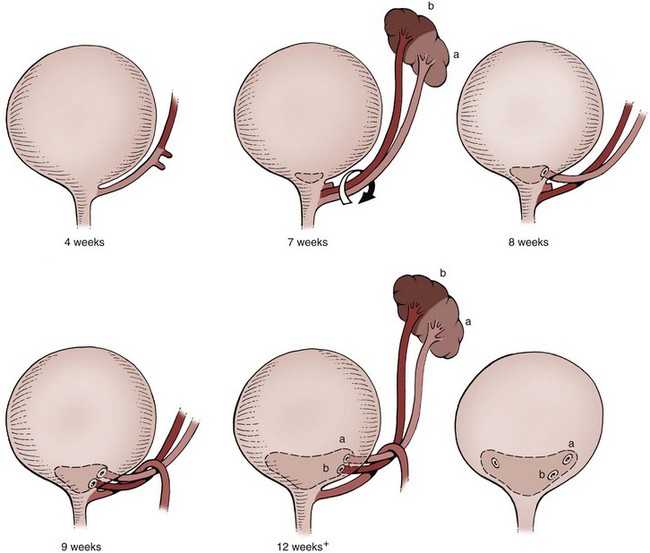
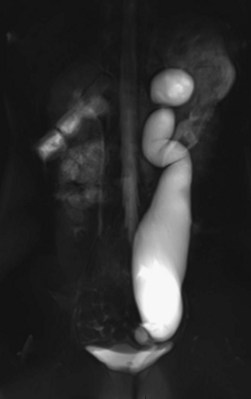
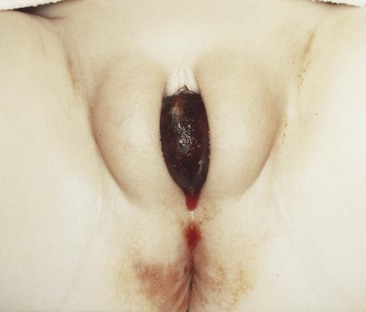
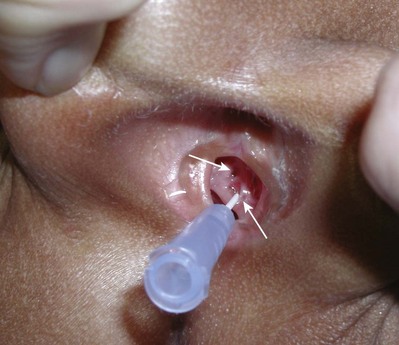
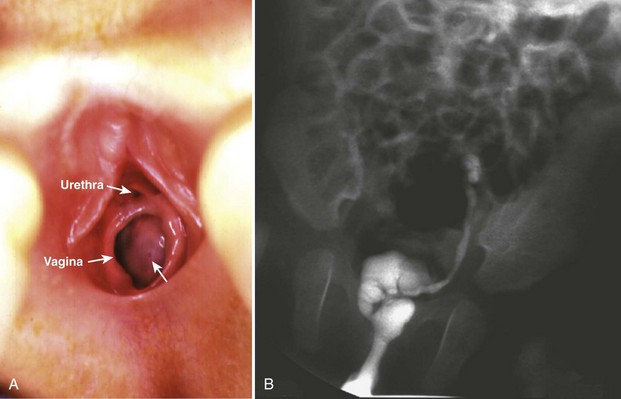
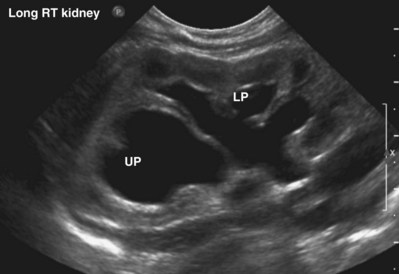
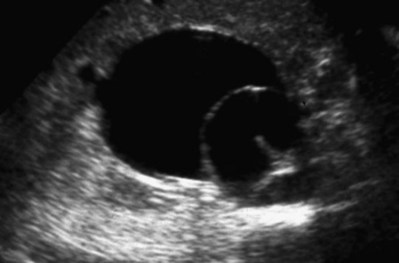
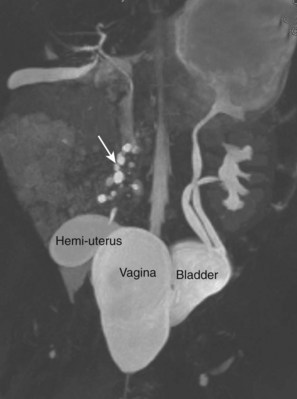
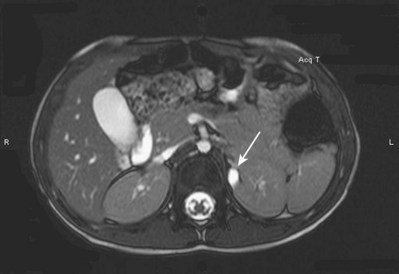
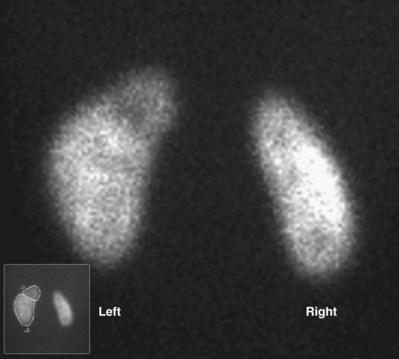
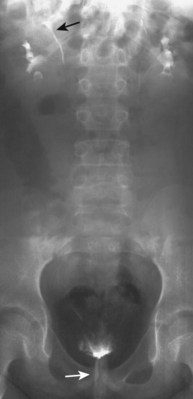
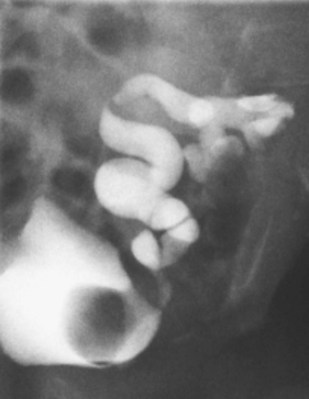
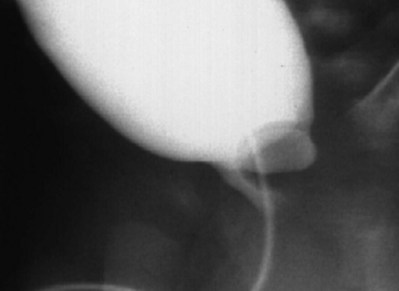
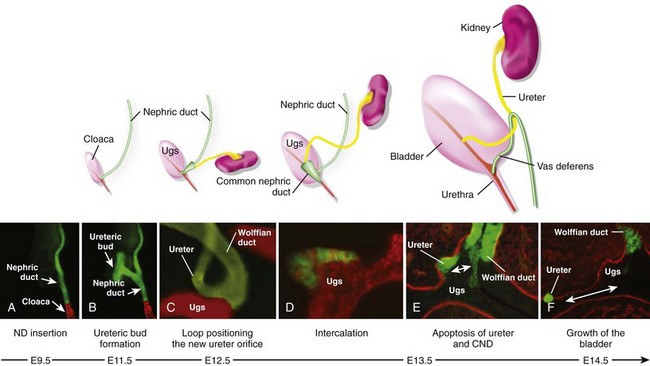
Craig A. Peters, MD, FACS, FAAP, Richard N. Schlussel, MD, Cathy Mendelsohn, PhD Abnormalities of ureteral development including ectopic ureters and ureteroceles represent a large component of clinical urology and continue to challenge clinical management, despite their wide recognition and well-defined surgical strategies. The wide spectrum of involvement and the variable patterns of presentation underlie the clinical challenge and require a thorough understanding of both normal and abnormal embryology of the lower urinary tract. This chapter presents the embryology, clinical manifestations, pathology, evaluation, and management strategies for ectopic ureters and ureteroceles, as well as other less common ureteral anomalies of formation. Obstructive ureteral pathologies including ureteropelvic junction (UPJ) obstruction and ureterovesical junction obstruction are reviewed in Chapter 120. By definition, an ectopic ureter is any ureter, single or duplex, that does not enter the trigonal area of the bladder (Glassberg et al, 1984). In a duplex system this is inevitably the upper pole ureter, presumably due to its budding from the mesonephric duct later than the lower pole ureteral bud. If it is simply near the bladder neck, it is not technically ectopic, which requires entry at the level of the bladder neck or distal. In females the ectopic ureter may enter anywhere from the bladder neck to the perineum and into the vagina, uterus, and even rectum (Weight et al, 2006). It may be associated with a cyst of Gartner duct, the remnant of the wolffian duct from which the ureter buds, and may include cystic dilation of the duct. The duct typically runs parallel to the vagina (the müllerian structure) and with rupture of the cystic ductal structure, communication with the vagina is established (Weiss et al, 1984; Rosenfeld and Lis, 1993). This is the basis for incontinence, the frequent presentation of an ectopic ureter in females. In males the ectopic ureter always enters the urogenital system above the external sphincter or pelvic floor and usually into the wolffian structures including vas deferens, seminal vesicles, or ejaculatory duct. These may be associated with cystic dilation, presumably due to continued urine production. Males do not present with incontinence but with infection and pain of the affected organs (testicles and epididymis). Single-system ectopic ureters may present in a similar fashion but may also be associated with an apparently absent kidney. This usually reflects an inability to identify the kidney on typical imaging and has led to the use of computed tomography (CT) imaging with contrast to detect small poorly functioning renal units associated with an ectopic ureter (Borer et al, 1998). Either single or duplex systems with an ectopic ureter may present with severe hydronephrosis reflecting distal obstruction. This may have impaired normal renal development to the point that the affected segment is nonfunctional, which needs to be clinically assessed. A rare entity of bilateral single-system ectopic ureters occurs and may be associated with a hypoplastic bladder and bilateral renal abnormalities, typically dysplasia (Noseworthy and Persky, 1982; Johnin et al, 2007). Some of these children may be considered to have bladder agenesis due to the absence of a recognizable bladder structure, presumably due to the absence of bladder filling and emptying in utero (Weight et al, 2006). Key Points Ectopic Ureter Ureteroceles may be seen to represent a version of the ectopic ureter with a cystic dilation of the distal aspect of the ureter that is located either within the bladder or spanning the bladder neck and urethra. Studies in mouse models suggest that ureteroceles may reflect defective ureteral maturation, the process by which the developing ureteric bud detaches from the mesonephric duct and moves to the bladder (see later) (Mendelsohn, 2009). As with the ectopic ureter, ureteroceles may be associated with a single or duplex system and in duplex systems they are associated with the upper pole. Ureteroceles can extend into the urethra but do not form entirely within the urethra, nor do they attach to the wolffian ductal structures. Several classification systems exist for ureteroceles, but the most useful one for clinical practice separates intravesical from extravesical ureteroceles. The intravesical ureterocele is entirely within the bladder and above the bladder neck. This would include a “simple” ureterocele that may be seen in the adult with minimal dilation and mild to no upper tract dilatation. This term, however, should be discouraged and the term single intravesical ureterocele used instead (Glassberg et al, 1984). An ectopic ureterocele includes those “in which some portion of the ureterocele is situated permanently at the bladder neck or urethra” (Glassberg et al, 1984). The orifice may be in the bladder, at the bladder neck, or in the urethra. This should be distinguished from an intravesical ureterocele that prolapses into the urethra with voiding. Further descriptive subdivision of ureterocele types has been published, particularly by Stephens (Stephens, 1971; Stephens et al, 1996). These include cecoureterocele, stenotic, sphincteric, sphinctero-stenotic, blind, and nonobstructed ureteroceles (Figs. 121-1 and 121-2). From a clinical perspective, the most important subgroup to recognize is the cecoureteroceles. In these cases the orifice of the affected ureter is within the bladder, but the cavity of the ureterocele extends beyond the bladder neck into the urethra. These may not be readily identified preoperatively (Smith and Parrott, 1994) and their complexity may create surgical challenges, particularly with endoscopic incision (see later). An unusual but diagnostically challenging ureterocele variant is the so-called “nonobstructive” ureterocele with duplication (Bauer and Retik, 1978) or “ureterocele disproportion” (Share and Lebowitz, 1989). These are associated with a duplex kidney, but the affected upper pole is nondilated and typically dysplastic to such a degree that it is not readily detected on most imaging and the affected ureter is not dilated beyond the bladder. A typical-appearing ureterocele is seen in the bladder, but the ipsilateral kidney appears normal. There is some association of ureterocele type with outcomes of various therapeutic measures, but these have not proven to be as predictable as initially reported. Churchill and colleagues (1992) proposed a classification system on the basis of the impact of the ureterocele on the upper urinary tract including all renal units. This functional system separates those in which the upper pole only is in jeopardy, from those in which an entire ipsilateral kidney is threatened, to those in which the contralateral system is also at risk due to reflux or bladder outlet obstruction. The latter group comprised 26% of the reported cases. The specific mechanisms responsible for ectopic ureters and ureteroceles remain undefined, but our emerging understanding of normal and abnormal ureterotrigonal development, along with new investigational tools, are likely to provide valuable insights (Mendelsohn, 2009). Although these molecular mechanisms will be of scientific importance, they will also permit potential separation between variants of these conditions that are likely to have therapeutic relevance, as well as perhaps ultimately permitting early detection (i.e., in utero) and intervention. The latter is a distant but not implausible horizon. At present experts remain uncertain as to fundamental mechanisms and yet need to know which ureteroceles and ectopic ureters may be associated with bladder neck and trigonal maldevelopment sufficient to cause incontinence. These children will require more aggressive therapy to achieve continence. Early identification may permit earlier and more effective surgical therapies. Classical understanding of uretero-trigonal development has indicated that the trigone originates from the mesodermal CND, as does the ureter, and is distinct from the endodermal origin of the bladder. According to this model, the CND expands, moving the ureter away from the mesonephric duct, and is incorporated into the primitive bladder, repositioning the ureter orifice at the bladder neck (Wesson, 1920; Tanagho and Pugh, 1963; Woodburne, 1965). Normal regulation of ureter remodeling permits fusion of the ureter and the UGS, as well as separation of the ureter and the wolffian duct (Mendelsohn, 2009; Tanaka et al, 2010). However, the observation that the CND degenerates during ureter maturation suggests that it may not give rise to the trigone as previously thought (Batourina et al, 2002, 2005; Viana et al, 2007; Chi et al, 2009; Mendelsohn, 2009; Uetani et al, 2009; Tanaka et al, 2010). Consistent with this, lineage studies and tissue recombination studies in mice suggest that the trigone is formed by bladder mesoderm/muscle and muscle surrounding the intravesical ureter. One possibility is that intersection between ureteral fibers and bladder muscle give the trigonal region its distinct morphology. A revised model of ureteral bud–trigonal maturation based on these studies requires coordinate expansion and subsequent apoptosis of the CND between the developing ureter and wolffian duct. Expansion of the CND permits correct alignment of the ureter with the UGS and may be seen to determine the position of the attachment of the ureter to the bladder and trigone. In contrast to the classic model, however, in which the CND forms the trigone, the revised model includes regression of the CND in the normal animal through apoptosis (Batourina et al, 2002, 2005; Viana et al, 2007; Chi et al, 2009) (Fig. 121–3). Figure 121–3 Top: schematic showing a revised model of ureter maturation. Bottom: images of Hoxb7-Gfp expressing embryos showing different stages of urinary tract morphogenesis. In the lower urinary tract, the Hoxb7-Gfp transgene is localized in the ureter, mesonephric (wolffian) duct, and common nephric duct (CND) (Srinivas et al, 1999). A, Whole mount urogenital tract from an E9 Hoxb7-Gfp embryo counterstained with E-cadherin to reveal the mesonephric duct (green) inserting into the primitive bladder (red). B, A whole mount urogenital tract from an E11 Hoxb7-Gfp embryo counterstained with E-cadherin (red). The CND lies below the primary ureteric bud branch, which at this stage has invaded the kidney blastema (not visible). C, Whole mount urogenital tract from an E12 Hoxb7-Gfp embryo (green) stained with cytokeratin (red). Apoptosis of the CND has brought the caudal-most ureter into close contact with the bladder, where it aligns, setting the position of the future ureteral orifice. D, A vibratome section from an E13 Hoxb7-Gfp urogenital tract stained with E-cadherin (red). Cells from the most distal ureter epithelium have now merged with the bladder epithelium, where they will undergo apoptosis, creating a new ureteral bud connection. E, A whole mount urogenital tract from an E13.5 Hoxb7-Gfp embryo stained with Laminin to reveal the basement membrane of the bladder epithelium (red). The ureter orifice is now inserted in the epithelium and is separated from the mesonephric duct by a short distance. F, Expansion and elongation of the bladder has moved the ureter orifice farther away from the mesonephric (wolffian) duct. Ugs, urogenital sinus. The expansion of the CND that permits normal alignment of the developing ureter appears to be in response to contact with the UGS (Batourina et al, 2005) and must be coordinated with later apoptosis of the CND, as well as being regulated by local factors and CND cellular responsivity. Impaired expansion may produce ectopia due to delayed or limited movement of the ureter relative to the UGS, whereas unregulated expansion may prevent normal communication of the ureter with the UGS and perhaps be the initiation of terminal ureteral expansion as seen in ureteroceles. Tanagho (1976) postulated that ureteroceles resulted from the distal ureter being inappropriately exposed to the factors inducing expansion of the UGS and bladder. Nearly all growth during development is the result of balanced stimulatory and inhibitory factors, regulated by temporal and spatial gradients, proximity, and cofactors that are, in turn, regulated by similar mechanisms. Altered timing and coregulation of these systems are likely the basis of altered development. The classic explanation of ureterocele formation is that of Chwalle (1927), postulating that distal ureteral expansion is produced by failure of rupture of a distal membrane at the ureteral orifice (Chwalle membrane). Obstruction is unlikely to be a major and certainly not the only factor producing ureteral dilation, as seen with ureteroceles. Ureteroceles with large orifices and no apparent obstruction are well recognized clinically. Ectopic ureters with insertion into the bladder neck are dilated but do not have the disproportionate distal dilation characteristic of the ureterocele. Obstruction plays some role in ureterocele pathophysiology, however, as is evident in the frequent occurrence of decompression with ureterocele puncture. Whether this is determined by the degree of muscularization of the ureterocele is undefined. Recent studies support the idea that degeneration of Chwalle membrane is important for generating a patent connection between the ureter orifice and the bladder. These studies suggest that Chwalle membrane, which has been thought to form from the UGS, may in fact be derived from luminal cells in the ureter that undergo apoptosis at a relatively late stage, before the onset of renal function, which may be important for generating a patent connection between the distal ureter and bladder. The possible sites of ectopic ureteral insertion are shown in Figure 121–4, including the common locations that are associated with normal wolffian duct development; however, more aberrant locations have been reported. It is unclear what the embryologic mechanisms of these locations, including rectum, might be, but they probably reflect more diffusely deranged overall development. Given that the wolffian duct in the male is never associated with any structures below the pelvic floor and sphincter, males do not present with urinary incontinence, as is seen in females. Abnormal bladder function is recognized in children with ureteroceles and is characterized by larger than normal bladders with inadequate emptying (Abrahamsson et al, 1998). This is potentially consistent with not only the obstructive effect the ureterocele can have on the bladder outlet, but also abnormal bladder and trigone development. As the developing ureteral buds incorporate into the developing UGS, the lowest bud, associated with the lower pole of the kidney, is incorporated first and migrates more laterally. The upper pole ureter is later to be incorporated and thereby is closer to the bladder neck. The Weigert-Meyer rule (Fig. 121–5) (see also Chapter 111) describes the inverse relationship of the duplex ureteral orifices, in which the ectopic ureter or ureterocele associated with the upper pole is caudal to the lower pole ureteral orifice. The association of the upper pole ureter and renal segment with the abnormally developed distal ureter further supports the principle that ectopic ureters and ureteroceles result from aberrant temporo-spatial signaling as the CND merges with the UGS. The majority of ureteroceles and ectopic ureters are detected through prenatal ultrasound (US) imaging, even if the specific diagnosis is not made. The abnormality prompts postnatal imaging, which will invariably determine the specific cause, induce further studies, and permit an adequate characterization of the condition. The prenatal imaging patterns are identical to those seen postnatally on US imaging yet may be misinterpreted. These are described in Chapter 114, but several elements should be emphasized here. The ectopic ureter can appear identical to a ureterocele with a dilated upper pole, tortuous ureter, but no intravesical component. On occasion, a large ectopic ureter may impinge on the bladder and appear as an intravesical structure, termed a pseudoureterocele (Sumfest et al, 1995). These patterns are identical to those seen postnatally but do require an experienced maternal-fetal ultrasonographer to recognize. With a tentative diagnosis of a ureterocele or ectopic ureter made, careful evaluation of the other renal units and bladder should be made. Ipsilateral lower pole or contralateral dilatation suggests reflux or less commonly obstruction from the ureterocele or the dilated ectopic ureter. Bladder outlet obstruction by a ureterocele can occur and present with hydronephrosis of all renal units. It is unusual to identify bladder obstruction to the extent to produce oligohydramnios, but this has been reported (Gloor et al, 1996; Crane, 2000) including in-utero decompression (Quintero et al, 2001; Ashmead et al, 2004). Contralateral renal dysplasia may be evident and associated with reduced amniotic fluid. The need for prenatal intervention or early delivery is exceptional and unlikely to provide any significant benefit. When significant hydronephrosis is present with either an ectopic ureter or ureterocele, an incidental diagnosis will occasionally be made. In some cases, however, this may result from a search for an explanation of general abdominal pain that was not considered to be due to a renal cause. It is also possible that the dilated ureter may not be recognized as such, and cases of presumed ovarian cysts that were actually markedly dilated ureters have been seen (Fig. 121–6). Although there is great variability in the presentation of either ectopic ureter or ureterocele, several patterns may be anticipated. In either case, generalized urosepsis may be the presenting clinical scenario and a renal bladder US will usually provide the diagnosis. It has been reported that an acute US study is unnecessary if a child presents with a febrile urinary tract infection (UTI) and has a history of a normal prenatal US after 30 to 32 weeks (Hoberman et al, 2003). The extreme variability in quality of prenatal imaging makes this recommendation tenuous, and it would seem prudent that an US be obtained in all children with urosepsis. In those with a febrile UTI, there is rarely urgency if they are clinically responding. The value of early detection is the potential for early treatment, which may be a simple drainage procedure. If the child is recovering rapidly, imaging may be elective. In the male, a similar subacute pattern of infection may be present, but more often, these boys present with epididymitis. Although this may be an unusual subset of young boys with an acute scrotum, they will have a true bacterial epididymitis and may or may not have infected urine. In the setting of a suspicion for epididymitis in a young boy, it is prudent to perform a brief US of the upper tracts to ensure no abnormality. Older males will also present in this manner, although it is unclear why it may be so delayed. Some have had recurrent episodes of epididymitis or scrotal abscess before the underlying cause is detected. Xanthogranulomatous pyelonephritis in the upper pole segment of an infant has been reported as well (Perez et al, 1999). In older children in whom the diagnosis has not been recognized, attribution of the symptoms may be due to dysfunctional voiding, laziness, and even sexual abuse (Heidemeier et al, 2007). During the history taking, signs of typical voiding dysfunction such as voiding postponement, posturing, and constipation should be sought to assess the likelihood of these being an explanation for the wetting. Ureterocele prolapse is an unusual but distinctive presenting sign that may still confuse the clinician. These are usually smooth, congested, mucosa-covered intralabial masses, and the child may be experiencing difficulty voiding. The mass protrudes from the urethra, distinct from the vagina, and is not circumferential as a urethral prolapse will be (Fig. 121–7). It is not lobulated as a sarcoma botryoides appears. An US of the bladder will usually confirm the diagnosis, and kidney images will further support this. Key Points Clinical Presentation With both ectopic ureter and ureterocele, physical findings may facilitate diagnosis. The prolapsed ureterocele is diagnostic and dramatic but unusual. Further study of the anatomy is necessary. It is unusual to be able to detect an ectopic perineal ureteral orifice in a child, but this will provide much information as to the character of the situation and best treatment (Fig. 121–8). A search for the perineal orifice in a child with continuous wetting or a known leaking ectopic ureter is worthwhile. Rarely, the dilated Gartner duct cyst may be detected on careful perineal examination (Fig. 121–9). These orifices or cystic structures may then be imaged by injection of contrast. Continuous dripping or pooling of urine may be seen in the introitus of females. Visualizing the actual orifice in the introitus is difficult due to the many mucosal folds. However, absence of dripping of urine does not exclude the diagnosis of ectopic ureter. The ectopic ureter may be connected to renal tissue so dysplastic that it rarely produces urine. The US image will usually provide the anatomic diagnosis and permit inference of functional assessment. The typical findings are as with the prenatal imaging of a dilated upper pole with ureteral dilation or a dilated single system (see Fig. 121–9). The character of the renal parenchyma will be more readily seen postnatally, and if it appears healthy, the true need for functional upper tract imaging can be questioned. Depending on the chosen treatment algorithm, it may be argued that this is unnecessary because it will not change treatment. The difference between functioning upper poles and those with little to no function are usually apparent (Figs. 121-10 and 121-11). Figure 121–10 Ultrasound image of dilated upper pole (UP) associated with a ureterocele, demonstrating limited renal parenchyma (same patient as Fig. 121–30). LP, lower pole. Bladder views should be diagnostic to differentiate ureterocele from ectopic ureter (Fig. 121–12). The ureterocele is characterized by a thin-walled, cystic dilation within the bladder and not extending beyond its walls. The laterality of the ureterocele is usually apparent but may appear midline if large. A lobulated ureterocele may appear as two structures, but careful examination should show communication. It is difficult to visualize extension of a cecoureterocele into the bladder neck, so nonvisualization is not diagnostic. Several US patterns may produce confusion in diagnosis. A large ureterocele may completely fill a bladder with no urine in the bladder itself. A full bladder with an effaced ureterocele may be mistakenly considered an ectopic ureter. Observation of the bladder over time will usually avoid these errors. A ureterocele may also evert and has been seen outside the bladder and mistakenly diagnosed as a diverticulum. A dilated ectopic ureter may produce an impression on the bladder and appear as a ureterocele (the previously noted “pseudoureterocele”) as noted in fetal diagnosis, but the wall will be clearly bilaminar and much thicker than typically seen with a ureterocele. Further, the lumen of the ectopic ureter will extend well outside the bladder walls (Fig. 121–13). (B, Courtesy of Dr. Jeanne Chow, Assistant Professor of Radiology, Harvard Medical School and Children’s Hospital Boston.) MR urography can provide the most detailed images of an affected urinary tract as a whole along with functional information yet is rarely useful to provide more data than may be obtained with less expensive methods not requiring sedation. Eventually, however, MR imaging (MRI) is likely to become the modality of choice. At present the value of MRI rests with cases in which other imaging cannot define complex anatomy. This may occur with massive dilation in which the existence of duplication may be uncertain or in which anatomic relationships are significantly distorted (Fig. 121–14). Not all ectopic ureters are massively dilated, and the MR can also be helpful in identifying a small, dysplastic upper pole and its ectopic ureter that was unrecognized on US. If this type of anatomic delineation is necessary, MR offers the addition of functional information that is an equally important aspect of evaluation. The ability to integrate detailed anatomic assessment of MR with functional assessment can be useful in the detection of the occult ectopic ureter causing incontinence with minimal function (Fig. 121–15). Radionuclide renal imaging remains the gold standard for renal functional assessment, and this is usually best provided by dimercaptosuccinic acid (DMSA) imaging. The function of the affected upper pole is the principal focus, but the health of the other renal moieties must be determined as well, particularly if there is lower pole reflux or hydronephrosis of any unit. Although there have been some attempts to define what the “normal” function of an upper pole associated with an ectopic ureter or ureterocele should be, there are no criteria to define this. The clinical impact comes with the decision to preserve the upper tract or not, yet there are no objective parameters to determine what level of functional contribution should be preserved. The functional assessment therefore offers the dichotomy of “some function” versus “no function,” and this may or may not determine clinical management (see later) (Figs. 121-16 and 121-17). In general, this is clear, but there will always be ambiguous cases. Preoperative assessment is further confused by the lack of ability to predict the effect of decompression. Figure 121–16 Dimercaptosuccinic acid renal scan in a child with a left ureterocele demonstrating no function in the affected upper pole. The assessment of drainage function is rarely of practical utility in that a purely observational approach is only rarely entertained and usually not on the basis of functional parameters. There are no guidelines as to the level of obstructive effect that will cause a clinical problem, particularly infection, although this has been used as a selection tool for nonoperative management (Han et al, 2005). In unusual situations the intravenous pyelogram (IVP) may offer adequate functional information with anatomic delineation without the complexities of MRI. Because the quantitative measure of upper pole function is rarely necessary with any precision, the IVP can offer an efficient means to complete the renal functional assessment (Fig. 121–18). The IVP may be of particular value in the ectopic ureter without ureteral or upper pole dilation. A simple bladder US image may be useful in completing the functional evaluation of these patients. This requires some patience to wait for bladder filling and emptying yet will yield information on bladder capacity, emptying efficiency, trabeculation, and bladder wall thickness (indicative of possible outlet obstruction). The character, location, and size of the ureterocele can usually be assessed as well (see Fig. 121–12). In some cases the ectopic nature of a dilated ureter may be identified ultrasonographically (see Fig. 121–13A). At times, the distinction between a ureterocele and ectopic ureter may be difficult, as discussed earlier. The presence of reflux may determine initial treatment for some practitioners and is an important parameter in clinical management after initial decompression of the ureterocele. Lower pole reflux may be of any degree and can often be associated with a massively dilated ureter (Fig. 121–19). In the setting of an ectopic ureter, ipsilateral lower pole reflux is unlikely to resolve spontaneously and will determine definitive treatment options. The appearance of the bladder base with filling and voiding demonstrated on VCUG will also be useful in therapeutic decisions because massive eversion indicates a weak trigonal floor that may be more likely to require surgical repair. Prolapse of a ureterocele into the urethra and demonstration of a cecoureterocele are important factors in planning and performing a TUI. These factors may be more predictive of the subsequent need for secondary surgery. The usual descriptive terms in the context of a ureterocele include effacement or flattening of the ureterocele with bladder filling; prolapse, which is movement of the ureterocele into the urethra; eversion; diverticulum-like protrusion of the bladder base and trigone with filling and voiding (Fig. 121–20); and intussusception, or protrusion of the ureterocele into its ureter (Fig. 121–21).
Classification and Anatomic Description
General Patterns
Ectopic Ureter
Ureterocele
Embryology and Etiology
Ureteral-Trigonal-Renal Development
Relationship with Embryonic Ductal Structures
Patterns Associated with Duplication
Clinical Presentation
Imaging
Prenatal Detection
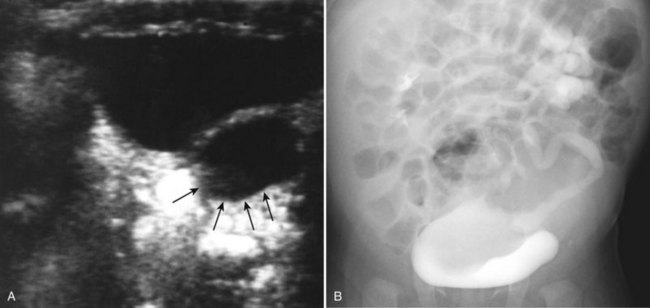
Incidental
Infection
Clinical Patterns
Incontinence
Clinical Patterns
Prolapse
Anatomic Assessment
Physical Examination
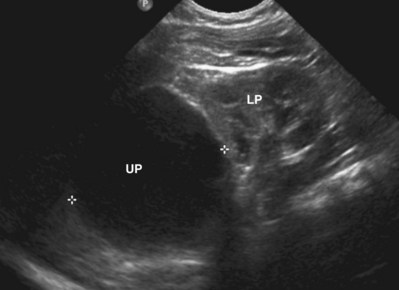
Ultrasound
Magnetic Resonance Imaging
Renal Function
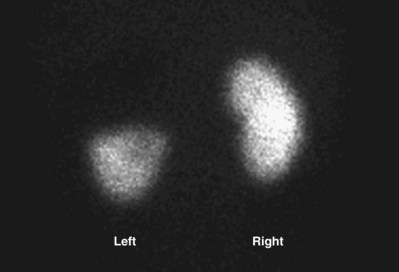
Nuclear Imaging
Intravenous Pyelogram
Bladder Function
Ultrasound Imaging Interpretation
Reflux
Ureterocele and Bladder Outlet