Warren T. Snodgrass, MD
Embryology of Penile Development
The external genital anlage is initially indifferent and develops the female phenotype unless exposed to androgens during the critical gestational time period of 8 to 12 weeks. 5α-Reductase type 2 is highly expressed in mesenchymal stroma while the androgen receptor is concentrated in epithelium of the urethral plate (Kim et al, 2002). Dihydrotestosterone derived from 5α-reduced testosterone mediates the key steps in penis formation: elongation of the genital tubercle and fusion of urethral folds.
The urethral plate develops as an extension of endoderm from the cloaca along the ventral midline of the genital tubercle. Proliferating mesenchyme to either side creates urethral folds and establishes the urethral groove. Fusion of the urethral folds begins proximally and continues distally at least to the glans. Two theories are proposed for glanular urethra development: ectodermal ingrowth cannulating the glans to the urethral plate (Glenister, 1954) versus urethral plate tubularization to the tip of the glans (Kurzrock et al, 1999).
Embryo studies suggest the penis initially exhibits ventral curvature (VC) during formation, which can persist in hypospadias when normal development arrests (Kaplan and Lamm, 1975). This VC originally was ascribed to fibrous tissue bands. However, histology from both embryos and resected surgical specimens demonstrate well-vascularized tissues without fibrosis (Baskin et al, 1998; Snodgrass et al, 2000).
Neither the molecular mechanisms involved in normal penis development nor those disturbances resulting in hypospadias are currently known. In mice, Sonic hedgehog (Shh) expressed by distal urethral plate epithelium is needed for genital tubercle outgrowth, with targeted deletion resulting in penile and clitoral agenesis (Haraguchi et al, 2001). Shh regulates fibroblastic growth factor-8 (fgf8), also known as androgen-induced growth factor, which upregulates expression of fgf10 involved in glans morphogenesis. Fgf10 also is expressed in midline mesenchyme during urethral fold fusion (Haraguchi et al, 2000), and deficient mutant mice exhibit a hypospadias-like phenotype that Yucel and associates (2004) believe indicates its crucial role in distal urethral plate tubularization. Fgf receptor 2 (Fgfr2) similarly is expressed in the urethral plate and prepuce in mice, and mice lacking it manifest proximal hypospadias (Petiot et al, 2005). Androgen receptor antagonists result in loss of Fgf10 and Fgfr2 expression in the urethra and hypospadias phenotype, indicating these genes are downstream targets involved in urethral formation (Petiot et al, 2005). The B-subclass of cell-surface Ehp and ephrin molecules also plays an important role in murine urethral formation, with heterozygous mutant males demonstrating perineal hypospadias (Yucel et al, 2007).
However, extrapolation of these findings in mice to human development is currently uncertain. Although Baskin and coworkers (2004) state the murine urethra tubularizes proximally to distally by urethral fold fusion similar to humans, others describe canalization of the urethral plate without fusion of urethral folds in rats and mice (Uda et al, 2004).
Etiology
Genetic Factors
Familial aggregation is found in 4% to 10% of hypospadias cases, including first-, second-, and third-degree relatives (Calzolari et al, 1986; Harris and Beaty, 1993; Fredell et al, 2002; Schnack et al, 2008). A recent national registration-based study from Denmark (Schnack et al, 2008) included 1.2 million males with 5380 hypospadias cases, reporting hypospadias equally transmitted through maternal and paternal sides of the family, with recurrence risk ratios similar for twin brothers, brothers, and sons. Difficulties in establishing recurrence risks include heterogeneity in the severity of hypospadias in probands, reliance on family histories introducing recall bias, and inclusion of patients with unrecognized syndromal hypospadias (Harris and Beaty, 1993; Schnack et al, 2008). Despite these limitations, a consistent conclusion is that familial aggregation is best explained by genetic factors rather than environmental exposure (Calzolari et al, 1986; Stoll et al, 1990; Schnack et al, 2008).
Endocrinopathies
The pivotal role of androgens in normal penis development suggests endocrinopathies impacting hormone production or action may underlie hypospadias. Leydig cell dysfunction was implicated by findings of elevated basal luteinizing hormone and reduced testosterone response to human chorionic gonadotropin stimulation in prepubertal boys with hypospadias versus controls (Nonomura et al, 1984). Defects in the testosterone biosynthetic pathway, specifically, impaired 3β-hydroxysteroid dehydrogenase alone or with impaired 17,20-lyase or 17α-hydroxylase activity, were reported in proximal hypospadias (Aaronson et al, 1997) but not confirmed in subsequent studies (Feyaerts et al, 2002; Holmes et al, 2004). Although enzymatic assays for 5α-reductase type 2 activity in isolated hypospadias are normal, mutations in its coding gene SRD5A2 on chromosome 2 were reported in 9% of patients with hypospadias not found in controls (Silver and Russell, 1999). Androgen receptor gene mutations are considered a rare cause of hypospadias (Batch et al, 1993; Hiort et al, 1994; Allera et al, 1995; Sutherland et al, 1996) but were not detected by others (Feyaerts et al, 2002).
Problems linking disturbed androgen activity to hypospadias were summarized by Holmes and associates (2004): little evidence has been found to suggest that nonsyndromic hypospadias without other genital anomalies is associated with defects in testosterone production, its conversion to dihydrotestosterone, or androgen receptor activity. Furthermore, the autosomal recessive pattern of inheritance characterizing steroidogenic enzyme disorders does not correlate with the genetics of hypospadias.
Gene Mutations
Murine studies indicating androgen receptor activity regulates Fgf8, Fgf10, and Fgfr2 involved in urethral development have led to screening for defects in these candidate genes in patients with hypospadias. Among cases of nonsyndromic familial hypospadias variants have been found in FGF8 and FGFR2 not seen in normal controls (Beleza-Meireles et al, 2007a).
Estrogens play a role in male development, with specific nuclear estrogen receptors, predominantly ER2, found in proximity to the androgen receptor. Variants of ER2 influence serum testosterone levels and have been described in patients with hypospadias (Beleza-Meireles et al, 2007b). Further evidence implicating estrogen-related events in urethral maldevelopment was the finding that several estrogen-responsive genes are upregulated in hypospadias patients, including ACT3, Cyr61, CTGF, and CADD45β (Wang et al, 2007). Polymorphisms of ACT3 were subsequently reported associated with hypospadias, as were less common mutations (Beleza-Meireles et al, 2008).
Endocrine Disruptors
A hypothesis that natural or synthetic compounds exerting estrogen-like and/or antiandrogen effects could result in hypospadias potentially is supported by several observations. First are reports that the incidence of hypospadias is increasing in Western nations where exposure to industrial compounds is presumed widespread. In addition, pregnant animals exposed to various chemicals in pesticides exerting estrogen-like or antiandrogen effects produce male offspring with urogenital anomalies, including hypospadias (Gray et al, 2004). Similarly, an increased risk for hypospadias has been identified in males after assisted reproduction, possibly attributable to progesterone administered to mothers. Finally, there is recognition that the estrogen receptor influences androgen activity as mentioned earlier, suggesting exposure to estrogen-like compounds may explain abnormal masculinization in the absence of demonstrated defects in testosterone production, 5α-reductase type 2 activity, or the androgen receptor.
However, although human penis development is potentially vulnerable to endocrine disruption, to date xenobiotics have not been linked to hypospadias (Baskin et al, 2001; Safe, 2005). Furthermore, the reported increase in hypospadias that suggests environmental endocrine disturbance has been challenged by recent epidemiologic studies (see later).
Syndromes with Hypospadias
Nearly 200 syndromes are associated with hypospadias (Edery, 2007).
Deletion in chromosome 11q13 results in WAGR syndrome (Wilms tumor, Aniridia, Genital anomalies, mental Retardation), associated with hypospadias due to altered WT1 gene activity. One study screening for WT1 gene defects in boys with nonsyndromic hypospadias reported no mutations (Nordenskjold et al, 1999).
Hand-foot-genital syndrome is an extremely rare autosomal dominant condition due to mutations in HOXA13 on chromosome 7p14-15, resulting in bilateral thumb and great toe hypoplasia. Hypospadias in mice mutant for Hoxa13 also have loss of Fgf8 and bone morphogenetic protein-7 in the urethral plate (Beleza-Meireles et al, 2007a).
13q deletion syndrome is characterized by mental retardation, facial dysmorphia, imperforate anus, and hypospadias with penoscrotal transposition. The critical region mediating anorectal and genital anomalies has been localized to 13q33.1-34, containing 20 annotated genes including EFNB2 (Garcia et al, 2006). Partial loss of ephrin B-2 function altering ephrin signaling in mice is associated with hypospadias, as discussed earlier.
Epidemiology
Prevalence
In the 1800s both Bouisson and Rennes reported that hypospadias occurred in 1 of 300 males (Beck, 1917). Data from birth registries in the United States during the late 1960s demonstrated a similar incidence (Paulozzi et al, 1997). Subsequent studies in the 1970s and 1980s indicated an apparent doubling in hypospadias occurrence in the United States, Hungary, and England, with upward trends in Denmark, Norway, and Sweden (Paulozzi et al, 1997; Aho et al, 2000a; Toppari et al, 2001). A simultaneous increase in the ratio of severe to mild forms of the condition in the Metropolitan Atlanta Congenital Defects Program suggested the increase was due to growing incidence rather than to more frequent reporting of distal cases (Paulozzi et al, 1997). Together these data from national birth registries have been taken as evidence for the influence of environmental toxins.
However, a review of surgically treated cases versus live births in Finland from 1970 to 1986 found prevalence of hypospadias unchanged despite the apparent increase noted in the national malformation registry (Aho et al, 2000a). Further investigation suggested hypospadias was previously underreported in the Finnish national malformation registry, as well as in Swedish and Danish registries (Kallen et al, 1986). Therefore, the apparent increase in hypospadias rate in these countries may only indicate changes in reporting.
In addition, various national databases have differences in reporting requirements. For example, in Australia and Japan only cases with the urethral opening on the penile shaft, scrotum, or perineum are registered, omitting the more common coronal and glanular forms (Toppari et al, 2001). Similarly, a comparison between surgical registries and the National Congenital Anomaly System in England found significant disparity in hypospadias rates attributed to a decision in 1990 to exclude reporting of distal cases (Nelson et al, 2007). Consequently, data from such registries cannot be used to accurately determine either incidence of hypospadias or geographic variations in its occurrence.
Other Associations
Placental dysfunction in early pregnancy has been implicated in risk for offspring with hypospadias, given its role in hormone production. Significant associations have been found to low birth weight (<10th percentile), preterm birth (<37 weeks), prepregnancy maternal obesity and diabetes, and maternal hypertension (Porter et al, 2005; Nordenskjold and Frisen, 2007; Akre et al, 2008). Both decreased (<24 years) and increased (>40 years) maternal age have been reported (Porter et al, 2005; Akre et al, 2008). Males have greater risk when paired with another male, versus female, twin, but the observation that twin pregnancy is associated with hypospadias may be explained by low birth weight (Nordenskjold and Frisen, 2007).
Assisted reproduction is associated with an increased hypospadias risk, which has been attributed to hormonal manipulations during and after the procedures (Macnab and Zouves, 1991; Silver et al, 1999), as well as paternal subfertility and twin pregnancy (Wennerholm et al, 2000).
Diagnosis
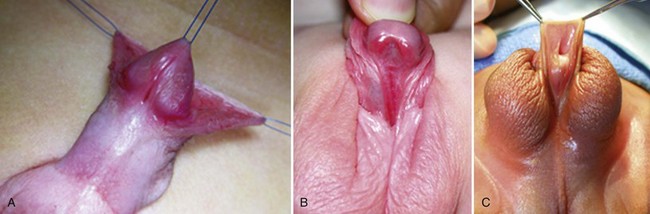
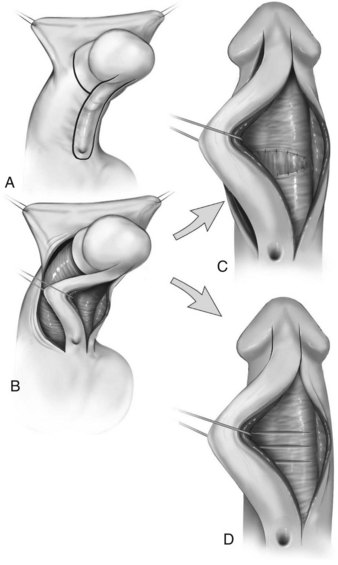
Hypospadias is diagnosed by physical examination, first suspected by the ventrally deficient prepuce and confirmed by the proximal meatus (Fig. 130–1). Other abnormal ventral findings potentially include downward glans tilt, deviation of the median penile raphe, VC, scrotal encroachment onto the penile shaft, midline scrotal cleft, and penoscrotal transposition.
A variant subset has a normally formed foreskin concealing a glanular to distal shaft hypospadias termed megameatus intact prepuce (MIP) (Fig. 130–2). Diagnosis is made after elective neonatal circumcision or in later childhood when the foreskin retracts.
Patients with ventrally deficient foreskins but a normally located urethral meatus are diagnosed as having chordee without hypospadias. The term implies ventral penile curvature, although, in the majority, apparent downward bending is corrected by simply degloving the ventral skin. This categorization has included patients with a glanular meatus but deficient corpus spongiosum and thin distal urethra that others consider hypospadias variants. To end confusion, boys with a hooded prepuce and bending should be diagnosed with congenital ventral curvature if the urethra is grossly normal or otherwise with hypospadias (Snodgrass, 2008).
Associated Anomalies
Cryptorchidism
Retrospective chart reviews (Kaefer et al, 1999; Wu et al, 2002) and a case-control study from the Danish National Patient Register (Weidner et al, 1999) report that approximately 7% of hypospadias patients also have cryptorchidism. In a series of 356 patients with hypospadias the incidence of cryptorchidism was 3.4% of 88 with distal versus 10% of 234 with proximal hypospadias (Wu et al, 2002).
Prostatic Utricle
An enlarged utricle sometimes hinders catheter placement during urethroplasty. After excluding patients with a disorder of sex development (DSD), urethrography demonstrates that enlarged utricles are uncommon in penile shaft hypospadias, with increasing incidence as severity progresses from penoscrotal to perineal cases (Devine et al, 1980; Ikoma et al, 1985).
Disorders of Sex Development
The simultaneous occurrence of hypospadias with cryptorchidism increases the likelihood for DSD. Overall reported incidence in patients considered to have a male-appearing phenotype ranges from 0% to 30% and is greater with increasing severity of hypospadias and nonpalpable testes (Rajfer and Walsh, 1976; Kaefer et al, 1999; McAleer and Kaplan, 2001; Cox et al, 2008). Kaefer and colleagues reported DSD in approximately 50% of patients with a nonpalpable testis and hypospadias.
Coexistence of hypospadias and cryptorchidism can be explained by other associations than DSD. For example, a case-control study examining risk factors independently for the two conditions within the same nationwide cohort in Sweden found low birth weight and prematurity positively correlated with each (Akre et al, 1999).
Malformation Syndromes
Key Points: Penile Development
Preoperative Evaluation and Management
Karyotyping
A karyotype may help categorize hypospadias as syndromic when there are other nongenital anomalies, especially developmental delay, dysmorphic facies, and/or anorectal or scrotal malformations. It may also detect gonadal DSD, especially when there is also cryptorchidism. The role for karyotyping in isolated hypospadias, even proximal cases, is unclear because most reports concern hypospadias associated with cryptorchidism (Rajfer and Walsh, 1976; Kaefer et al, 1999; McAleer and Kaplan, 2001; Cox et al, 2008) or do not state the severity of isolated hypospadias (Moreno-Garcia and Miranda, 2002).
Radiologic Studies
No prospective studies report the incidence of radiologically detected urinary tract anomalies associated with hypospadias. Initial retrospective reviews concerned intravenous pyelography, and of these the largest series found upper tract anomalies are not increased with nonsyndromic hypospadias (McArdle and Lebowitz, 1975; Lutzker et al, 1977; Cerasaro et al, 1986). Routine voiding cystourethrography to demonstrate an enlarged utricle is not necessary, because the most common clinical manifestation is difficult catheterization that can be managed intraoperatively. Imaging can be reserved for screening patients with suspected syndromic hypospadias or DSD.
Timing of Surgery
There are no data regarding optimal timing for hypospadias surgery in children, and so guidelines are derived from expert opinion. The 1996 action committee for the American Academy of Pediatrics Section on Urology reviewed psychological factors, anesthetic considerations, and technical aspects of hypospadias repair before recommending surgery be performed between 6 and 12 months, assuming the surgeon, anesthesiologist, and facility were experienced in the care of infants (American Academy of Pediatrics, 1996). Same-day surgery can be performed after 50 gestational weeks in otherwise healthy boys, leading the author to routinely recommend repair at 3 months of age or older for distal hypospadias and selected proximal cases with an apparently normal-sized phallus. Infants with proximal hypospadias and a small-appearing glans are reassessed at 3 months and then administered hormonal stimulation, as discussed later, before surgery at approximately 6 months of age.
Optimal timing for surgery in children presenting at an older age is uncertain. From 18 months to approximately 3 years of age has been described as a difficult period for hospitalization, leading to a recommendation that repair be postponed to age greater than 3 years (Manzoni et al, 2004). However, the American Academy of Pediatrics action committee observed that surgery from 30 to 65 months of age may increase the child’s anxiety for physical injury. One study compared health-related quality of life assessment after hypospadias surgery done at less than 18 months of age versus more than 18 months of age and found no differences related to age at operation (Weber et al, 2008). Preoperative sedatives, minimal separation from family, same-day surgery, anesthetic blocks that reduce early postoperative pain, dressings that fall off spontaneously, and urinary diversion into diapers all may reduce psychological stress from hypospadias repair.
Preoperative Hormonal Stimulation
One reported regimen is intramuscular testosterone enanthate 2 mg/kg given 5 and 2 weeks preoperatively (Gearhart and Jeffs, 1987; Luo et al, 2003). A comparison between a mixture of testosterone propionate and enanthate providing a dose of 2 mg/kg/wk administered twice daily topically or intramuscularly weekly found no differences in response regarding penile length or diameter. Elevated serum levels greater than 10 ng/mL only were noted after topical therapy, possibly from excessive application (Chalapathi et al, 2003).
Reported mean increases in penile length range from approximately 0.5 to nearly 3 cm, with partial to complete loss of these gains by 3 to 12 months after stimulation (Gearhart and Jeffs, 1987; Davits et al, 1993; Chalapathi et al, 2003). Side effects include pubic hair growth and aggressive behavior; accelerated linear height and bone age have not been documented (Gearhart and Jeffs, 1987; Davits et al, 1993; Chalapathi et al, 2003), but few patients have had bone age assessed after stimulation.
The author prefers two to three intramuscular injections of testosterone enanthate 2 mg/kg over a 6- to 12-week period. Hormonal stimulation has been used in less than 1% of distal repairs versus 25% of proximal to perineal cases and is recommended when the glans appears small, recognizing there are no published criteria to define normal or adequate glans diameter (Snodgrass and Yucel, 2007).
General Aspects of Surgical Repair
Suture Materials
No randomized controlled trials (RCTs) compare suture materials while controlling other factors that may influence outcomes, including severity of hypospadias, type of repair, and suturing technique. Recommendations therefore reflect the surgeon’s preference. Available needles for various sutures may also influence choice and outcomes but are rarely reported in hypospadias literature. Sutures and needles preferred by the author are listed in Table 130–1.
Table 130–1 Sutures and Needles for Hypospadias Repair
| SUTURE SIZE AND MATERIAL | NEEDLE |
|---|---|
| 7-0 polyglactin | TG 140-8 |
| 6-0 polyglactin | S 29 |
| 4-0, 5-0 polyglactin | RB |
| 6-0 polypropylene | BV 1 |
| 6-0, 7-0 polydioxanone | BV 1 |
| 4-0, 5-0 polydioxanone | RB |
Perioperative Antibiotics
No RCTs have been performed to determine if hypospadias surgical outcomes are influenced by preoperative intravenous antibiotics. One RCT comprising 101 patients compared intraoperative intravenous cefonicid plus postoperative oral cephalexin to intravenous cefonicid alone. There were no differences in the two groups regarding surgical complications, but both asymptomatic bacilluria (21% vs. 51%, P < .05) and febrile urinary tract infection (6% vs. 23%, P < .05) were more common without postoperative antibiotics (Meir and Livne, 2004). Postoperative antibiotics are commonly used during urinary diversion; the author prefers trimethoprim/sulfamethoxazole.
Postoperative Urinary Diversion
One RCT randomized 64 toilet-trained boys (median age 6 years, range 2 to 17) undergoing tubularized incised plate (TIP) repair by a single surgeon to postoperative bladder catheterization or not, decided at the conclusion of the procedure. There were no differences in urethroplasty complications; however, dysuria (14% vs. 45%, P < .01), urinary retention (0% vs. 24%, P < .05), and urinary extravasation (0% vs. 17%, P < .05) occurred significantly more often in the unstented group (El-Sherbiny, 2003).
A prospective observational study by the same surgeon involved 32 consecutive non–toilet-trained boys (mean age 18 ± 6 months) undergoing TIP repair with no urinary diversion. Urinary extravasation developed in one patient the second postoperative day resulting in catheter placement. There were no urethroplasty complications with mean follow-up of 9 months (Almodhen et al, 2008).
Another prospective study compared 36 patients undergoing Mathieu repair with catheter (n = 17, mean age 4.1 years) versus without diversion (n = 19, mean age 4.6 years) and no difference was reported in urethroplasty complications at 6 months or longer follow-up (McCormack et al, 1993).
Dressings
Two RCTs, one having 100 patients operated on by one surgeon (Van Savage et al, 2000) and the other comprising 120 patients and four surgeons (McLorie et al, 2001), were done to evaluate the role of postoperative bandages. In the first study patients had an adhesive film versus no dressing, whereas the second compared postoperative topical antibiotic ointment alone to an adhesive film or compressive wrap followed by topical antibiotic ointment applied after dressing removal. In both, 2% of patients were excluded from randomization owing to bleeding at the conclusion of the procedure resulting in a bandage. Parents in both studies were instructed to remove the bandage by postoperative day 2 (Van Savage et al, 2000) or when it appeared loose (McLorie et al, 2001). Neither study reported the appearance of the penis on catheter removal (e.g., extent of edema or hematoma formation). Urethroplasty outcomes were not impacted by dressings versus no dressings. Both studies noted greater numbers of telephone calls from parents of boys with bandages, although one did not state if these calls specifically related to the dressing.
Historic Background
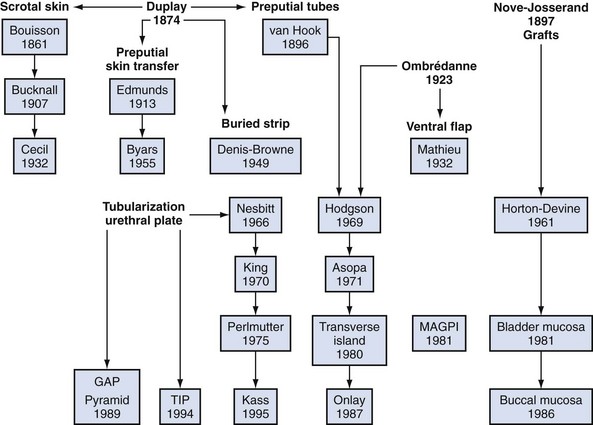
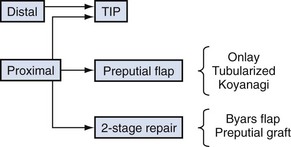
Although it is commonly written that more than 200 repairs have been described, hypospadias urethroplasty can be summarized into three basic categories: tubularization of the urethral plate, supplementation or substitution of the urethral plate with skin flaps, or urethral plate substitution with grafts (Fig. 130–3). Of these, today the most common primary surgery is urethral plate tubularization. This is also the most recently introduced concept, given that the urethral plate—tissues extending distally from the hypospadias meatus that normally form the urethra—was only recognized as a distinct structure in the late 1980s.
Modern hypospadias surgery dates to 1980. Duckett (1980) mobilized inner preputial flaps on a vascular pedicle away from dorsal shaft skin, providing greater flexibility to transpose them ventrally. The following year he described the meatoplasty and glanuloplasty (MAGPI) technique for glanular defects (Duckett, 1981), opening the door for routine distal repair that today comprises most hypospadias surgery. It was also Duckett who defined the urethral plate and then modified his tubularized preputial flap (“transverse island”) to the onlay version supplementing, rather than substituting, the plate after realizing routine “chordee” excision was not necessary (Baskin et al, 1994). By the end of the decade nearly all primary hypospadias could be repaired using MAGPI, onlay, or tubularized preputial flaps.
Although tubularization of the broad or deeply grooved urethral plate had been reported sporadically, in 1994 the midline incision was described to tubularize the narrow or flat plate without the need for supplemental skin flaps (Snodgrass, 1994). The TIP repair subsequently gained widespread use for its perceived simplicity and improved cosmetic outcomes versus flap repairs. Its application to proximal hypospadias followed increased realization that the urethral plate is not fibrous scar but well-vascularized tissues that potentially can be preserved for urethroplasty, even in some cases with VC.
Hypospadias Surgery
Ventral Curvature
Etiology
The penis exhibits VC during normal development (Kaplan and Lamm, 1975), which implies that persistent bending in hypospadias reflects arrested development. Consequently, ventral tissues—including shaft skin, dartos, corpus spongiosum, urethral plate, and overlying tunics of the corpora cavernosa—may be shortened relative to the dorsal surface. Previous concepts that dysplastic dartos and corpus spongiosum coalesce into fibrous “chordee” tissues that require resection have been disproved by histologic studies in both embryos and surgical specimens referenced earlier (Baskin et al, 1998; Snodgrass et al, 2000).
Extent of Curvature
Prevalence and severity of VC after release of shaft skin varies by extent of hypospadias. From the author’s experience since 2000, VC occurred in 50 (11%) of 440 primary distal cases undergoing artificial erection (Snodgrass et al, 2010), 12 of 40 (30%) midshaft repairs (Snodgrass and Yucel, 2007), and 57 of 70 (81%) proximal repairs (Snodgrass and Prieto, 2009). VC in all patients with distal and midshaft repairs was less than 30 degrees.
It is also important to consider what extent of VC impairs sexual activity. Reports concerning adult men seeking medical attention suggest bending 30 degrees or more is present before sexual dysfunction occurs (Bracka, 1989; Thiounn et al, 1998; Van Der Horst et al, 2004; Greenfield et al, 2006).
Preoperative and Intraoperative Assessment
Cephalad traction on the glans stay suture causes the bent (>30 degrees) penis to pivot from side to side. Such observations formed the basis for determining VC and its correction until saline corporeal injection was described by Gittes and McLaughlin in 1974. Artificial erection induced by saline injection remains the most commonly used means to assess presence and severity of VC, as well as to document successful correction. Criticisms of the technique include the potential for supraphysiologic or subphysiologic intracorporeal pressure during injection to distort findings. After ventral dissection of tissues from the tunica albuginea and/or ventral lengthening procedures for the corpora cavernosa (see later discussion), repeat erection may be difficult, owing to corporeal leakage.
Vasoactive drugs have also been injected to induce erection during hypospadias repair (Perovic et al, 1997; Kogan, 2000), with the proposed advantage being more physiologic assessment in contrast to saline injection. However, dose regimens are empirical in children, erection lasts longer than with saline irrigation unless a reversal agent is injected, and there may be increased bleeding from the prolonged erection.
Straightening Maneuvers
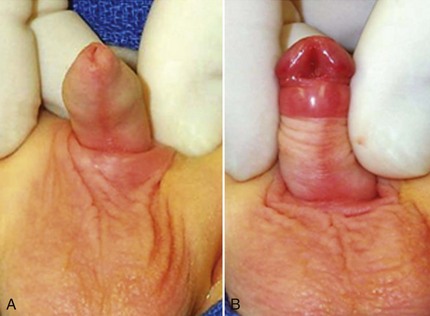
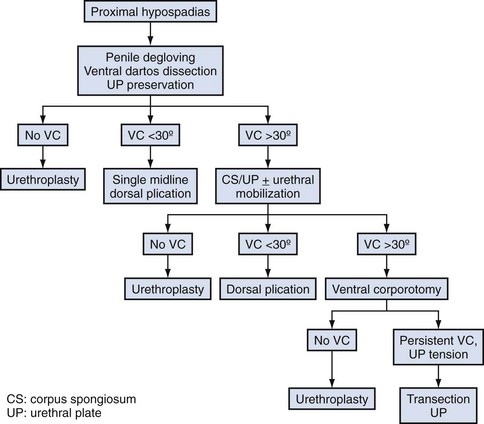
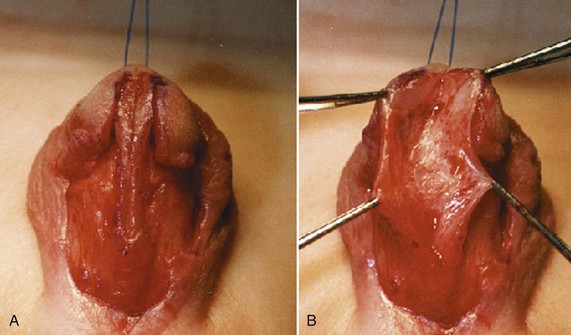
Curvature up to 30 degrees can be corrected by midline dorsal plication into the tunica albuginea of the corpora cavernosa directly opposite the area of greatest bending (Fig. 130–4). Baskin and colleagues (1998) reported lack of sensory nerves in this location, and straightening without corporotomy can be achieved.
There are few reports concerning sutures used, efficacy, or durability of plication, and assessment is complicated by lack of objective determination of intraoperative and postoperative extent of curvature. The only outcomes data for midline plication to date are found in a retrospective review of 43 prepubertal boys, 53% with VC greater than 30 degrees, whose curvature was straightened using one or two polypropylene 4-0 or 5-0 sutures. At median 16 months’ follow-up, 93% of patients were thought to maintain a straight penis (Bar Yosef et al, 2004). In contrast, the author uses only a single 6-0 polypropylene suture for midline plication of bending less than 30 degrees, preferring alternative methods to multiple plications when curvature is greater.
Similarly, optimal management of VC greater than 30 degrees remains uncertain. Multiple plications using 5-0 to 4-0 polypropylene are described, although concerns for both recurrent curvature and loss of penile length increase when more than one plication is performed (Braga et al, 2008). This extent of bending after skin degloving and ventral dartos dissection is considered disproportion between the ventral and dorsal aspects of the corpora cavernosa (Baskin et al, 1994). Ventral lengthening traditionally involves transection of the urethral plate followed by transverse incision from the 3- to 9-o’clock position into the tunica albuginea to expose erectile tissues of the corpora in the region of greatest curvature. The resultant defect has been closed using dermal grafts, small intestine submucosa, or tunica vaginalis flaps or grafts (Fig. 130–5). No evidence indicates the outcome is significantly influenced by choice of materials for corporotomy repair. Most proponents of the use of small intestine submucosa have used single-layer material; use of four-layer small intestine submucosa was reported in two small series and was associated with recurrent curvature in one (2 of 12 cases) (Soergel et al, 2003) but not in another (0 of 21 cases) (Elmore et al, 2007).
Urethral plate transection to facilitate corporeal grafting requires substitution urethroplasty using preputial flaps or grafts in either one or two stages. Alternatively, the urethral plate and adjacent corpus spongiosum can be preserved and dissected from the underlying surface of the corpora cavernosa. Elevation of the plate alone has been reported to correct curvature (Mollard and Castagnola, 1994), or it can be combined with corporotomy and grafting (Kajbafzadeh et al, 2007). Dissection under the urethral plate can also be continued proximally to near the membranous junction, elevating the normal urethra from the corpora (Fig. 130–6). This maneuver was adapted from urethral stricture repair in which mobilization of the urethra combined with its inherent elasticity allows gaps to 5 cm in adults to be bridged (Warwick et al, 1997). After dissection of the urethral plate and urethra, residual VC can be corrected by dorsal plication or ventral corporotomy (Riccabona et al, 2003; Bhat, 2007; Snodgrass and Prieto, 2009). Rather than a single corporotomy with grafting, two to three transverse incisions can be made into the tunica albuginea without exposing erectile spongy tissue or need for grafting (see Fig. 130–5) (Devine, 1983; Snodgrass and Prieto, 2009). The preserved urethral plate can then be incorporated into TIP repair or onlay urethroplasty.
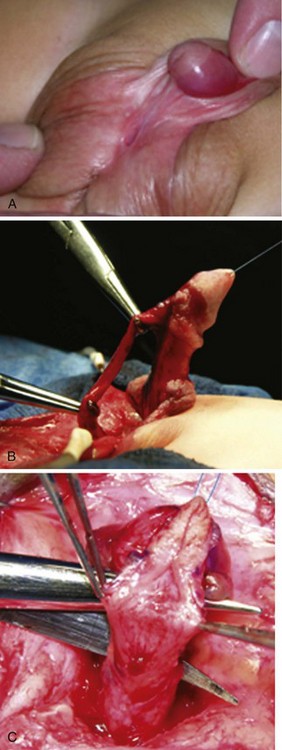
(Reprinted from Snodgrass W, Prieto J. Straightening ventral curvature while preserving the urethral plate in proximal hypospadias repair. J Urol 2009;182[Suppl. 4]:1720–5.)
VC that persists despite mobilization of the urethral plate and urethra requires urethral plate transection for straightening. In the author’s experience 15 patients with penoscrotal to perineal hypospadias have undergone urethral plate elevation alone (3 patients) or combined with urethral mobilization (12 patients), of which 3 patients had persistent bending despite dissection to the membranous region, visibly due to tethering by a short urethral plate (Snodgrass and Prieto, 2009).
A proposed algorithm for correcting penile curvature is outlined in Figure 130–7.
Congenital Penile Curvature
Description of patients diagnosed with “chordee without hypospadias” is complicated by varying inclusion or exclusion of patients with divergent corpus spongiosum and a hypoplastic urethra. In series excluding such cases, VC is attributed most often to deficient ventral shaft skin and dartos, followed by corpora cavernosa disproportion, with a shortened urethra the least common finding (Donnohoo et al, 1998; Tang et al, 2007).
Outcomes of Straightening after Puberty
No report describes effects of pubertal growth on straightening achieved by dorsal plication. A single series comprising 16 patients, 14 with proximal hypospadias and 2 with congenital penile curvature without hypospadias, provides information after corporotomy and dermal grafting (Badawy and Morsi, 2008). Although all patients were postpubertal at final review, only 3 were sexually active. Fifteen reported firm erection, but 1 patient undergoing repair after puberty subsequently noted impaired erection despite sildenafil, requiring intracorporeal injection for sufficient firmness. The authors reported weakness and bulging at the site of his graft.
Key Points: Ventral Curvature
Urethroplasty
The most commonly used options for primary hypospadias urethroplasty are summarized in Figure 130–8. It is traditional to describe repairs in separate categories for distal, midshaft, and proximal defects based on meatal position after skin degloving and correction of VC, although an alternative classification would be to consider primary repairs accomplished by tubularizing the urethral plate versus options when the plate is not maintained for urethroplasty (Sozubir and Snodgrass, 2003), most notably when VC greater than 30 degrees results in plate transection.
Distal Hypospadias
The most commonly performed operation to repair distal hypospadias is the TIP repair. Although other procedures such as MAGPI, Mathieu flip-flap, and urethral advancement remain in use, a survey of current practices indicates these together account for less than 10% of distal procedures (Cook et al, 2005). Technical aspects of these operations are little changed from prior descriptions in this textbook, with the exception of V incision into the ventral lip of flip-flaps, which attempts to create a vertical meatus (Boddy and Samuel, 2000). However, a study correlating preoperative configuration of the urethral plate (deep vs. moderate or shallow groove) to cosmetic results after V incision into onlay preputial flaps questions the reliability of this maneuver to achieve a vertical neomeatus unless the plate already is deeply grooved (Hayashi et al, 2007).
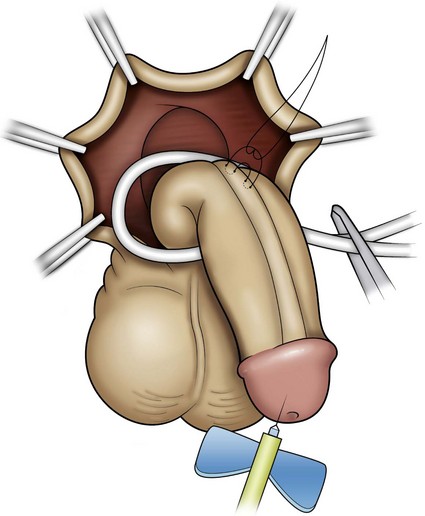
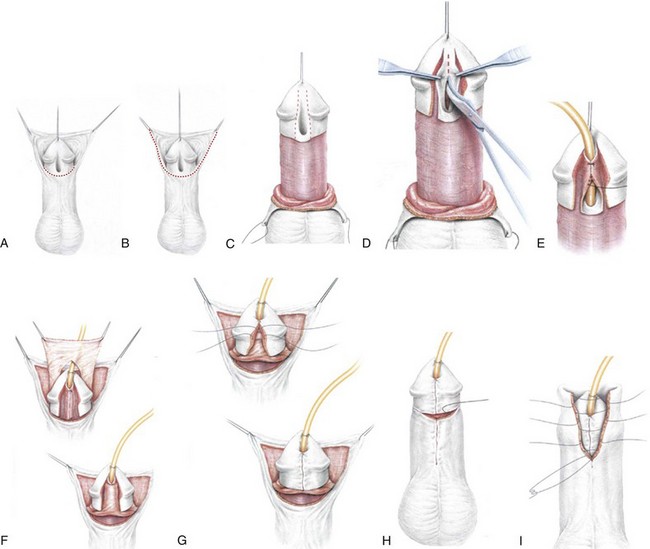
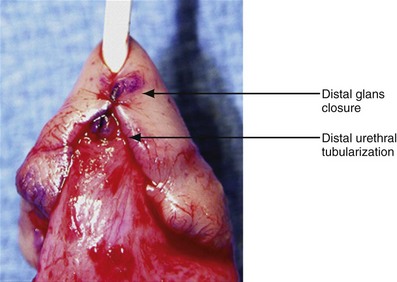
Figure 130–9 depicts TIP repair. The key step is midline incision of the urethral plate, which widens a narrow plate and converts a flat into a deep plate groove (Fig. 130–10), ensuring a vertical, slit neomeatus and a normal-caliber neourethra. Tubularization should not continue beyond approximately the midpoint of the dissected glans wings, corresponding to a point some 3 mm proximal to the distal extent of the plate, to avoid iatrogenic meatal stenosis. Glansplasty approximates the glans halves distally to create a normal-appearing meatus independent of plate tubularization (Fig. 130–11), because the opening of the urethral plate is not sutured to the glans as is commonly done with flaps.
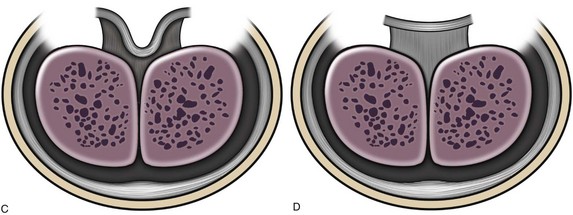
(A and B, Reprinted from Snodgrass W, Koyle M, Manzoni G, et al. Tubularized incised plate hypospadias repair: results of a multicenter experience. J Urol 1996;156[2 Pt. 2]:839–41; C and D reprinted from Snodgrass WT. Tubularized incised plate hypospadias repair: indications, technique, and complications. Urology 1999;54[1]:6–11.)
Contraindications
Whether urethral plate characteristics (flat vs. deeply grooved, narrow vs. wide) determine the appropriate use of TIP repair has been debated. Some consider a flat and/or narrow urethral plate a contraindication to TIP repair (Holland and Smith, 2000), whereas others report TIP repair reliably corrects distal hypospadias regardless of urethral plate configuration (Nguyen and Snodgrass, 2004). The author has not found an alternative procedure necessary for distal hypospadias repair in over 500 consecutive cases (Snodgrass et al, 2010).
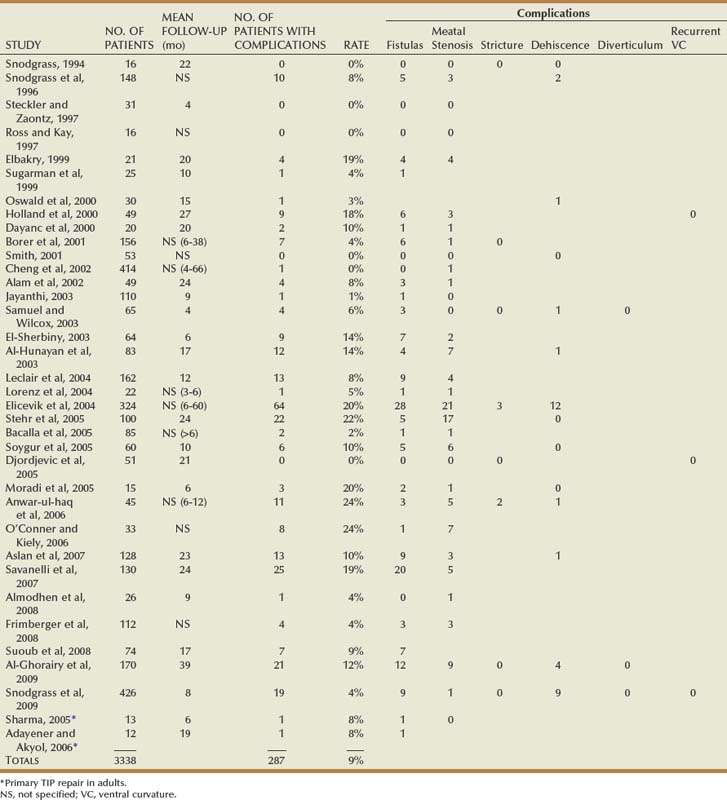
Outcomes
Table 130–2 summarizes published short-term results for TIP distal urethroplasty. The longest reported mean follow-up is 2 years, and to date no series describes outcomes after puberty in patients operated on when children.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree