Contemporary radiologic imaging has resulted in an increasing number of smaller renal cortical tumors being identified. The ability of imaging to classify these tumors is limited, although certain features may help classify the renal cortical neoplasm. The important role of radiologic imaging in tumor detection, characterization, staging, and follow-up of patients who have renal cortical tumors is reviewed in this article.
The renal cortical tumors are a family of neoplasms that are subdivided into benign and malignant neoplasm based on the Heidelberg classification, which more accurately predicts the metastatic potential of the tumor. The malignant renal parenchymal tumors include the clear cell renal carcinoma, papillary renal cell carcinoma, chromophobe renal cell carcinoma, collecting duct (Bellini duct) carcinoma, and a small number of unclassified tumors. The benign renal parenchymal tumors include renal oncocytoma, metanephric adenoma, metanephric adenofibroma, and papillary renal cell adenoma.
The different renal cortical tumors are associated with different disease progression and metastatic potential. The conventional clear cell carcinomas have the greatest metastatic potential, whereas papillary and chromophobe carcinomas are associated with less metastatic potential. The oncocytoma is virtually benign.
Detection
CT is considered the modality of choice for detection and diagnosis of renal cortical tumors. MR imaging and ultrasonography (US) are problem-solving tools or are used in patients who have contraindications to IV contrast. The improvement in radiologic imaging has led to a great increase in the detection and earlier diagnosis of renal cortical tumors. Currently greater than 70% of tumors are discovered incidentally and are generally small, with a median tumor size of less than or equal to 5 cm. Up to 20% of the suspicious renal cortical tumors detected may be benign (such as oncocytoma, angiomyolipoma [AML], or complex cyst).
Renal US is not considered a useful screening modality because small lesions can be easily missed. CT detects more and smaller renal masses than does US but the two modalities are comparable in characterizing 1- to 3-cm lesions. Although renal sonography may not be the best method for generalized primary screening it may still be beneficial in secondary screening in a more selected patient population, such as the elderly asymptomatic population.
Unfortunately, currently imaging has limited criteria for diagnosing the specific type renal cortical tumor without operative resection. This article specifically focuses on the techniques and roles of CT, US, and MR imaging in the evaluation of renal cortical tumors. The specific renal cortical neoplasms discussed in this article are clear cell renal carcinoma, papillary renal cell carcinoma, chromophobe renal cell carcinoma, renal oncocytoma, and AML.
CT Scan
A specific renal protocol is ordered to work up a known or suspected renal lesion. At our institution, a CT scan dedicated for evaluation of a renal mass typically consists of three imaging series: precontrast, corticomedullary phase, and late nephrographic/early excretory phase. Precontrast images are used for identification of calcifications and fat, and to provide a baseline density Hounsfield unit (HU) measurement for evaluating the degree and pattern of enhancement in cystic or solid renal masses. Nonionic intravenous (IV) contrast is injected through an IV line. Corticomedullary images are used for the identification of the renal lesion and assessment of lesion vascularity, renal vascular anatomy, and tumor involvement of venous structures. They are probably the most informative for lesion characterization. Not all renal tumors are well identified during the corticomedullary phase and the images obtained during a later phase of enhancement (ie, the nephrographic or excretory phase) are included for the detection of renal masses, especially those of smaller size. These excretory phase images are also helpful for identification of anatomic abnormalities or tumor involvement of the renal collecting system. High accuracy (sensitivity up to 100%, specificity up to 95%) has been reported in the detection of renal cortical tumors using proper technique.
The key features in characterizing a renal lesion on CT are: cystic versus solid, enhancement (attenuation change from the noncontrast to the contrast-enhanced images), margins, presence and type of calcification, and the presence of fat. There are strict CT criteria in characterizing a lesion as “simple” and therefore a benign cyst. The cyst must be well marginated, nonenhancing, homogeneous low attenuation (0–20 HU), and have a thin smooth wall. If the lesion on the precontrast study has measurements greater than 20 HU it is not a simple cyst and may represent a complex cyst or a mass. A contrast-enhanced study must be performed to evaluate for enhancement.
Enhancement was considered a change of 10 HU after IV contrast when CT was first introduced and on the single detector scanner. Now with the advancement in CT technology and the use of multidetectors the assessment of enhancement is more complicated. One common pitfall in characterizing renal lesions by CT is the presence of pseudo-enhancement in renal cysts on contrast-enhanced CT images. This finding is believed to be attributable to volume averaging and beam-hardening effects, and the degree of pseudo-enhancement is greater in smaller renal cysts. A change of more than 15 HU is considered by some to indicate enhancement. Others consider a change of 10 to 20 HU to be indeterminate and in need of further evaluation. Enhancement must be unequivocal because it implies that the lesion has a blood supply, is solid, and is considered a renal cortical tumor after the additional imaging features are considered. For lesions with indeterminate enhancement and in those patients for whom IV contrast is contraindicated US or MR imaging can be performed.
Generally speaking the presence of calcifications in a solid renal mass indicates malignancy. Calcification in a complex cyst with no enhancing soft tissue elements is not concerning for malignancy.
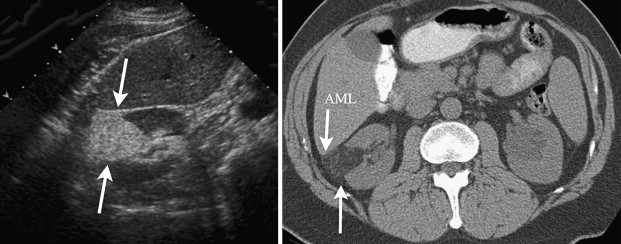
The presence of macroscopic fat implies benignity and the presence of an AML. Fat is diagnosed on the precontrast images. A low-density area, preferably within the center of the lesion, is selected by a region of interest and the HU are measured. If the HU are −10 or less, then fat can be confidentially identified ( Fig. 1 ). The fatty component needs to be in the center of the renal lesion. Large renal cortical lesions can grow and engulf the perirenal and sinus fat. Alternatively extrarenal lesions can grow centrally and invade the renal cortex giving the impression that the lesion is renal cortical in origin when it is not. As the name implies, a renal cortical tumor has its epicenter/origin from the renal cortex. An important role of imaging is to correctly identify the origin/location of a tumor. On imaging we look for a claw or beak sign of normal-appearing renal cortex at the periphery of the mass that indicates that the mass is renal cortical in origin. There are some caveats that need to be remembered. There have been reports of fat-containing renal cell carcinoma. Most have contained calcification. Renal cell cancer can rarely contain fat and no calcification. It is recommended that renal masses containing both fat and calcification should be considered malignant.
Exophytic lesions are significantly more likely than central lesions to be the less aggressive non–clear cell tumors and clear cell tumors are more likely to be central, implying that the exophytic lesion has a better prognosis. Central renal lesions may be urothelial in origin and it is important to alert the clinician of this possibility because this may affect management.
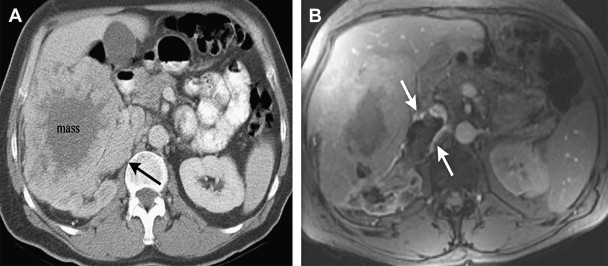
The renal veins and the inferior vena cava (IVC) are an important part of the evaluation in a patient who has a suspected renal malignancy. A thrombus involving the renal vein or IVC in a patient who has malignant renal tumor may represent tumor thrombus, blood clot, or both ( Fig. 2 ). The presence of enhancement within the thrombus indicates tumor thrombus, whereas bland thrombus would not enhance after contrast administration.
Solid renal cortical tumors
Enhancement is important in the characterization of solid renal cortical tumor.
A study at our institution by one of the authors (JZ) demonstrated that 90% of the clear cell cancers were hypervascular and heterogenous, 75% were of the papillary type and were hypovascular, and chromophobe often demonstrated moderate enhancement. A mixed enhancement pattern was most predictive of clear cell.
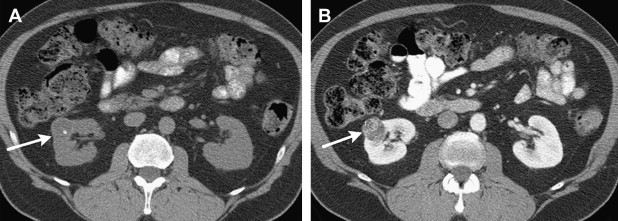
Clear cell carcinoma tends to be hypervascular and heterogeneous ( Fig. 3 ). Clear cell carcinomas demonstrate peritumoral vascularity more frequently than other malignant renal tumors of similar size.
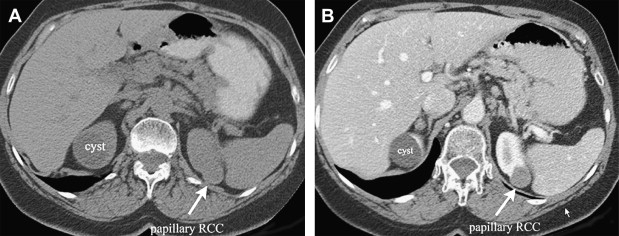
Papillary renal cell cancer is typically hypovascular and homogeneous ( Fig. 4 ).
The chromophobe is more variable in appearance. It may demonstrate moderate enhancement. The spoke-wheel–like enhancement with a central scar has been described as an important imaging feature.
Oncocytomas may overlap with renal cell carcinoma in imaging features and degree of enhancement. Classic angiographic findings for oncocytoma are a spoke-wheel pattern, a homogeneous tumor blush, and a sharp, smooth rim. But none of these findings are specific and a renal cell carcinoma may have any or all of the classic findings. The diagnosis of oncocytoma may be suggested if a central stellate scar is identified on CT within an otherwise homogenous tumor.
The presence of macroscopic fat is characteristic of an AML. AML may present without evidence of macroscopic fat—the so-called “AML with minimal fat.” AMLs have been described as being homogeneously high attenuation on the unenhanced study and demonstrated homogeneous enhancement. Overlap has been reported with renal cancer. Small (≤3 cm) homogeneously enhancing renal masses on CT may be an AML with minimal fat or renal cell carcinoma.
Cystic renal cortical tumors
The Bosniak Classification system grades the cystic renal masses based on CT findings for the likelihood of malignancy. Category 1 lesions are simple cysts. Category 2 lesions are benign minimally complex cysts that may contain thin septations with calcification. Category 2F lesions are more complex cysts with increase septations and calcification and require follow-up. Category 3 lesions are indeterminate masses with thick irregular walls and may contain calcification. When any solid enhancing component is present, the cystic renal mass is graded as Bosniak category 4 and considered malignant. The presence of nodular or septal enhancement has been shown to have the highest sensitivity for predicting malignancy with good to moderate interobserver agreement.
Ultrasound
US is an important tool in the evaluation of the renal cortical tumor. It can characterize a renal lesion as cystic or solid. When a lesion is described as cystic it is important to indicate if the lesion is a simple cyst and therefore benign, or a complex cyst and a possible surgical lesion. A simple cyst is anechoic: without echoes and black on the images. A simple cyst has a thin imperceptible wall, posterior enhancement, round or oval shape, and is avascular. If the cyst does not fulfill all the criteria of a simple cyst then it is complex, and the possibility of a cystic renal carcinoma may need to be considered depending on the sonographic features. Features on US suggestive of a malignant cystic lesion include a thickened (>2 mm) cystic wall, numerous septations, thickened or nodular septations (>2 mm), irregular or central calcifications, and the presence of flow in the septations or cystic wall on Doppler imaging. If any suspicious features are present correlation with other imaging modalities, such as CT and MR imaging, is recommended. Rarely a benign cyst can become complex in the setting of hemorrhage or infection, and could mimic a complex cystic lesion. In that setting comparison with prior imaging and history is critical.
Most renal cortical tumors on US are solid and described by their echogenicity. The terms used to describe the echogenicity are anechoic, isoechoic, hypoechoic, and hyperechoic. A simple cyst is anechoic. The renal cortical tumors may be hyperechoic, isoechoic, or hypoechoic relative to the normal renal cortex ( Fig. 5 ). A hyperechoic mass has more echoes and is brighter/lighter than the normal/adjacent renal cortex. An isoechoic lesion is the same as the adjacent renal parenchyma and the hypoechoic mass has fewer echoes and is darker. Cystic areas and calcifications may be present.
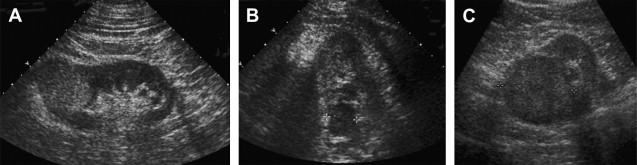
All solid renal masses on US usually need to be evaluated with a renal protocol CT for the presence of fat. If fat is present in a noncalcified lesion on CT then in most cases an AML can be diagnosed. AML is typically intensely echogenic and can cause acoustic shadowing. Every echogenic mass in the kidney must be further evaluated with a renal protocol CT to confirm the presence of fat (see Fig. 1 ). Up to one third of small renal cell cancers (<3 cm in diameter) can be markedly hyperechoic and mimic AML. Two percent of the renal cancers larger than 3 cm can be markedly echogenic. Alternatively, not all AMLs are hyperechoic and some are only diagnosed after surgical excision.
Attempts have been made to differentiate renal cortical tumors into the different histologic subtypes based on US characteristics. Papillary renal cell carcinomas tend to be hypoechoic or isoechoic but some may also be hyperechoic.
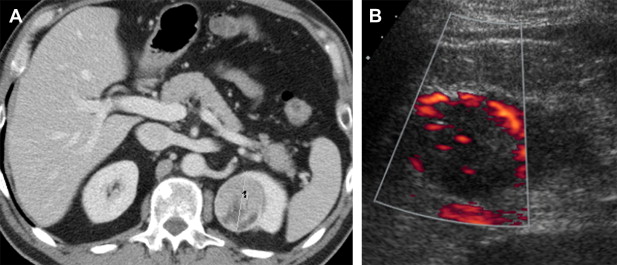
Work has been done in US using Doppler imaging, which allows for assessment of vascular flow. A recent study from our institution indicates that vascular flow within a renal mass, identified by color or power Doppler, is strongly associated with conventional clear cell carcinoma ( Fig. 6 ). Contrast-enhanced US has been found to be useful in the diagnosis of renal cortical tumors and in the detection of tumor blood flow in hypovascular renal masses. Contrast-enhanced US has also been useful in classifying renal cystic lesions into the Bosniak classification.
US and cross-sectional imaging modalities (CT and MR) complement each other in the characterization of renal lesions. In the subgroup of patients whose renal lesions are indeterminate on US, a dedicated renal protocol CT or MR imaging may help further characterize the lesion. Conversely, US may prove useful for renal lesions that are considered indeterminate on CT.
US may also assist in the preoperative evaluation of renal cortical tumors. Renal US is requested by the urologists as a template for the intraoperative US. It assists in the selection of the proper surgical technique and determining if a partial nephrectomy can be attempted. Because of its multiplanar capability US can demonstrate important landmarks and planes of sections that can be duplicated in the operating room.
MR Imaging
MR imaging has distinct advantages over other imaging modalities in the detection and staging of renal neoplasms, because of its intrinsic high soft tissue contrast and direct multiplanar imaging capabilities. In addition, pseudo-enhancement artifacts that frequently afflict CT examinations are typically not present on MR images. MR imaging may be used, therefore, to definitively determine the presence or absence of contrast enhancement in a renal mass that poses as a diagnostic problem on CT. Compared with US, MR imaging is not as operator dependent, nor is its image quality as susceptible to the patient’s body habitus. There are certain limitations to MR imaging, however, because of its relatively long acquisition time, greater cost, and thus limited access. In addition, MR imaging used to be considered safe in patients who had renal failure because gadolinium-based contrast agents were believed to be renally excreted yet nonnephrotoxic. MR imaging was often performed as the modality of choice for patients who had renal dysfunction yet needed contrast-enhanced cross-sectional imaging. Unfortunately, an association between administration of gadolinium-based contrast agents and nephrogenic systemic fibrosis, a rare but potentially disabling or even fatal sequela, has been recently established in patients who had renal failure undergoing MR imaging. In patients who have moderate to severe renal dysfunction, therefore, the current recommendation is that administration of gadolinium-based contrast agents should be performed with caution and alternative imaging modalities should be considered.
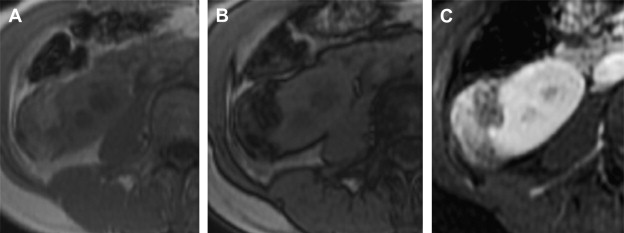
A modern MR imaging protocol aimed at evaluating renal masses typically includes the following breath-hold sequences: (1) a T1-weighted in- and opposed-phase gradient echo sequence, which is helpful in identifying macroscopic and microscopic fat in a renal mass ( Fig. 7 ); (2) a T2-weighted half-Fourier single shot fast spin echo sequence in axial or coronal planes, which is useful for evaluating the overall anatomy, renal collecting system, and the complexity of a cystic renal lesion; and (3) a dynamic contrast-enhanced T1-weighted fat-suppressed sequence for evaluation of the presence and pattern of enhancement in a renal mass. For the dynamic contrast-enhanced images, three-dimensional fast spoiled gradient echo sequences are typically performed before and after contrast administration during the arterial, corticomedullary, and nephrographic phases. Multiplanar reconstruction may be performed if necessary to better delineate the spatial relationship of the renal mass to adjacent anatomic structures. If necessary, a dedicated MR angiography sequence may be performed in the coronal plane during the arterial phase for better visualization of accessory renal vessels and facilitation of surgical planning. Coronal images may also be obtained during the excretory phase (often with administration of diuretics), from which maximum-intensity projection images can then be obtained to produce intravenous pyelogram–like images. For patients who cannot cooperate with breath-hold instructions, alternative T1-weighted sequences may need to be explored to reduce respiratory motion–related artifacts. For example, a two-dimensional T1-weighted MR sequence with an inversion recovery pulse followed by a long echo train may provide T1-weighted soft tissue contrast and shortened acquisition time to reduce image blur. The T2-weighted half-Fourier single shot fast spin echo sequences are more robust and less susceptible to motion artifacts, and can typically provide diagnostic images even in non–breath-hold patients.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








