Chapter 49 COMPLICATIONS OF INCONTINENCE PROCEDURES IN WOMEN
Stress urinary incontinence (SUI) is a common problem that affects millions of women in the United States. Various treatments exist, including physiotherapy, medications, devices, periurethral bulking agents, and more definitive surgeries. In contrast to less invasive therapies, surgery for SUI is associated with relatively high success rates. However, it also has potentially higher risks of morbidity and, rarely, mortality. Typically, surgery for SUI involves restoring support to the bladder neck, proximal urethra, or mid-urethra. Traditionally, anti-incontinence procedures have been broadly categorized as anterior repairs, transvaginal needle suspensions, retropubic suspensions, and sling procedures. More recently, midurethral synthetic slings, done in a minimally invasive fashion, have become the most popular procedures to treat SUI.
INTRAOPERATIVE COMPLICATIONS
Bleeding
A thorough history should uncover any bleeding diathesis. Significant bleeding is infrequent in either transabdominal or transvaginal approaches, occurring in 0.6% to 10% of cases.1 In one study,2 of 117 women who underwent Marshall-Marchetti-Krantz (MMK) cystourethropexy, 20 lost more than 500 mL of blood and 5 lost more than 1200 mL. Intraoperative bleeding during transabdominal approaches most commonly occurs in the retropubic space, owing to its rich network of venous complexes. If bleeding cannot be controlled by suture ligating, packing may be used.
During transvaginal procedures, bleeding commonly occurs at two points. The first is during the dissection of the vaginal wall off of the periurethral fascia. If this dissection is too deep, bleeding can be encountered. It is important to carry out dissection just above the glistening white surface of the fascia. This can be more difficult in cases of previous surgery, and greater care should be taken to ensure that the proper plane is dissected. The second point at which bleeding may occur is with perforation of the endopelvic fascia. This bleeding can be difficult to control, because the vessels responsible may not be directly visible. We have found that the best way to control this bleeding is to place the sling (or suspension sutures) and transfer them to the suprapubic region expeditiously. Also, a vaginal packing placed after closure of the vaginal wall helps to tamponade bleeding. The retropubic space is a contained space, and bleeding is usually self-limited. If vaginal packing is ineffective, a Foley catheter balloon has been reported to achieve transvaginal tamponade.3,4 Rarely, embolization may be done. Recently, Zorn and colleagues reported successful embolization of massive retropubic hemorrhage from a branch of the obturator artery injured during placement of a tension-free vaginal tape sling (TVT).5
With the widespread use of TVT and other midurethral slings, surgeons should be aware of problems related to bleeding specific to this procedure. Nilsson and Kuuva reported a retropubic hematoma rate of 3.3% and an additional 3.3% of patients with blood loss of greater than 200 mL.6 Flock and associates reported that a pelvic hematoma larger than 300 mL and requiring surgical intervention was found in 1.2% of patients.7 In 7 cases (2.1%), increased intraoperative blood loss (250 to 400 mL) was managed by cauterization, compression, or tamponade.7 Others have reported hemorrhage rates of 0.9% to 2.5% with TVT.8–10 In the Austrian registry of 2795 TVT procedures, 0.7% of the patients required laparotomy for bleeding control or evacuation of hematomas.11 Kuuva and Nilssons12 reported an incidence of 0.1% for injury to major vessels and nerves in their cohort of 1455 patients.
For the TVT procedure, the trocars are usually placed blindly, and knowledge of pelvic anatomy is crucial in avoiding disastrous complications. Deviation of curved trocars laterally can cause injury to the external iliac vein13 or artery14 and to the obturator neurovascular bundle. Cephalad displacement of the TVT trocar can cause injury not only to bowel but also to the iliac vessels more proximally. Patient movement during a TVT procedure peformed with local anesthesia can directly cause deviation from the intended path of the trocar. Anatomic dissections and cadaver studies have shown that the TVT trocars pass an average 4.9 cm from the external iliacs and 3.2 cm from the obturator vessels.15 With the transobturator (TOT) approach to placement of the TVT, the device passed on average 1.1 ± 0.4 cm from the most medial branch of the obturator vessels. The mean distance to the obturator nerve was 2.5 ± 0.7 cm.16 Currently, 30 cases of major blood vessel injury have been reported, including 2 fatalities.17 No significant bleeding complications have been reported with the TVT placed in a transobturator route. In most cases, bleeding after TVT procedures is self-limited and no specific intervention, other than perhaps transfusion, is required. However, in cases of large blood loss, open exploration or embolization may be necessary.5,11
Bladder and Urethral Injuries
If injury to the bladder, bladder neck, or urethra occurs as a result of needle or trocar passage or suture placement, it is usually recognized during cystoscopy, after suspension sutures or pubovaginal slings have been transferred. In our experience, this occurs more commonly in cases in which the needle is passed “blindly” rather than under direct finger guidance. If the injuries are small and are not associated with tearing, the sutures or instruments can simply be removed and replaced. The small perforation should heal with several days of catheter drainage. Larger injuries should be repaired with absorbable suture, and urethral tears should be treated with at least 5 to 7 days of catheter drainage. If a urethral injury occurs, absorbable suture may close the defect, depending on size and location. For TVT and similar devices, the trocar should be removed and another pass of the trocar should be performed. Bladder injury due to intravesical trocar passage may be treated with 48 to 72 hours of continuous bladder drainage with a Foley catheter. Bladder perforation from the TVT trocars occurs in 1.1% to 25% of TVT cases.12,18–22 The higher rates of bladder perforations occur in cases of previous failed anti-incontinence surgeries causing retropubic scarification. There may be a propensity for bladder perforations to occur opposite the side of the surgeon’s dominant hand.23
It is paramount to find intravesical TVT sling material during the initial placement, because secondary postoperative detection may require significant retropubic surgery. It is important to fill the bladder with at least 250 mL of irrigant or to near bladder capacity, because folds of mucosa may otherwise hide the tape. The 70-degree lens shows the anterolateral bladder wall, and the 0- and 12-degree lenses show the urethra optimally. Buchsbaum and colleagues24 reported on bladder injuries identified not by cystoscopy but by having high fluid/irrigant leakage from trocar or incision sites. Shobieri and coworkers25 reported a series of occult bladder injuries with TVT seen not on initial cystoscopy but only after removal and retrieval of trocars on second cystoscopy. In the TOT passage of synthetic midurethal tapes, case reports exist, and one series reported a 0.5% incidence of bladder perforations; hence, we recommend cystoscopy in this procedure as well.26,27 Using an inside-to-outside technique of TOT trocar passage, de Leval28 reported no instances of bladder perforation or visceral injuries in a series of 107 patients. The technique clearly holds promise, and further randomized studies to determine efficacy and complications of this technique should be performed.
Injuries occurring during transvaginal procedures that are closer to the bladder neck should be repaired primarily. These commonly occur when the vaginal dissection is done deep to the periurethral fascia and the surgeon attempts to perforate into the retropubic space. These injuries tend to be larger than trocar or needle perforations, and primary closure is usually recommended. If such an injury is found, a two-layer closure with absorbable sutures (e.g., polyglycolic acid) can usually be accomplished transvaginally. A drain can be placed in the retropubic space percutaneously from the suprapubic area, in much the same way that the suspension needle is passed, but this is rarely needed. Infrequently, a retropubic approach must be taken to ensure proper closure of a massive injury. If there is a history of prior pelvic radiation therapy in a patient who has sustained a bladder injury, tissue interposition (i.e., omentum, labial fat pat) is recommended.29,30
Ureteral Injuries
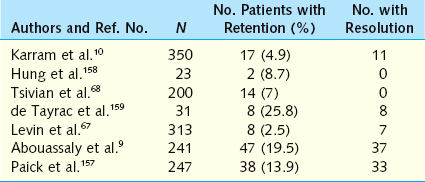
Injuries to the ureters are much less common than bladder and urethral injuries. They may be encountered in retropubic operations (e.g., MMK, Burch) as well as transvaginal procedures (needle suspensions and pubovaginal slings), and they usually occur near the ureterovesical junction.31 Rosen and colleagues,32 in a review article, reported 19 ureteral injuries in the literature secondary to Burch colposuspension (Table 49-1). It is important to emphasize that there may also be a risk of ureteral kinking with overcorrection, as described in the original article.33 Erikson and associates reported a 1% rate if unrecognized ureteral obstruction.34 Perforating injuries can be managed by removing the suture and stenting the ureter. If the injury is severe, ureteral reimplantation may be necessary. Ureteral injuries should be suspected if, on cystoscopy during anti-incontinence procedures, no efflux is seen from the ureteral orifices. If a delayed diagnosis of ureteral obstruction or laceration is made postoperatively, the patient may present with unexplained flank pain, fever, or drainage. An intravenous urogram, retrograde ureteropyelography, or computed tomographic urogram will usually delineate the injury. Immediate retrograde ureteral stenting is recommended. If this is not possible, stenting can be performed in an antegrade fashion. If this fails, open repair with removal of suture and sometimes ureteroneocystostomy are necessary. Ureteral injuries are uncommon with transvaginal procedures and usually involve the inclusion of the ureter in the suspension sutures. If this is recognized intraoperatively (when indigo carmine is not seen excreted from the ureter), the suspension sutures should be removed. It may be helpful to place a stent before replacing the suspension suture, but this is rarely needed. If such an injury is discovered after surgery, during the postoperative period, it is handled in the same fashion as described previously. We know of no cases of ureteral injuries associated with TVT.
Table 49-1 Ureteral Injury or Kinking with Burch Colposuspension
| Authors and Ref. No. | N | Ureteral Injury/Kinking (%) |
|---|---|---|
| Klutke et al.152 | 97 | 0 |
| Harris et al.153 | 60 | 6.7 |
| Eriksen et al.3 | 75 | 1.3 |
| Galloway et al.154 | 50 | 2 |
| Korda et al.155 | 174 | 1.1 |
| Petri156 | 1500 | 0.2 |
Other Injuries
If the peritoneum is inadvertently incised during either approach, it should be closed with an absorbable suture. Bowel injuries are rare but can occur with transfer of sutures or slings and with TVT and similar procedures. They may also occur with placement of a suprapubic tube. A small bowel laceration and small perforations of the colon below the peritoneal reflection can usually be closed primarily. However, perforations of the colon above the peritoneal reflection in unprepared bowel may require proximal colostomy if significant spillage occurs intraperitoneally. Bowel injuries have not been reported with the TOT procedure. The traditional TVT trocars passed retropubically may cause bowel injury when the trocars do not “shave” the posterior aspect of the pubis and are directed more cephalad. The risk of bowel perforation is small, but several cases have been reported.8,35–38 Patients with prior transperitoneal pelvic surgeries are at highest risk. Peyrat recommended preoperative computed tomography in women with a history of multiple abdominal and retropubic surgeries, which increase the risk of bowel adhesion to the pubis.39 Patients typically present with abdominal pain, peritoneal signs, or leakage of feculent material from suprapubic incision sites. High suspicion levels for bowel injury should be maintained for those patients complaining of abdominal pain after TVT. A small bowel obstruction caused by intraperitoneal TVT performed with concomitant vaginal hysterectomy for prolapse was reported.40
Sequelae of Intraoperative Injuries
Fistula
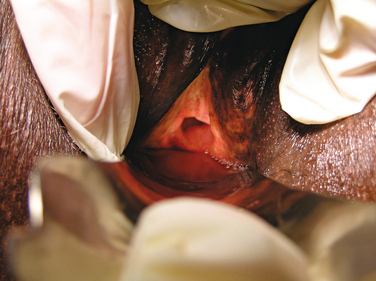
A fistula may develop after a surgical injury to the urinary tract. Unrecognized bladder or urethral injury may lead to a vesicovaginal or a urethrovaginal fistula, respectively (Fig. 49-1). If intraoperative cystoscopy is routinely used as described by Stamey,41 urethral and bladder injuries should be recognized, thereby decreasing the risk of subsequent fistula formation. Some instances of unrecognized injuries missed by cystoscopy may be detected when fluid leakage is more than expected from incision sites. Methylene blue or sterile infant formula may also be instilled into the bladder to aid detection. Kuuva and Nilsson, in a nationwide analysis of complications associated with TVT in 1455 patients, found only one case for an incidence of 0.7/1000 of vesicovaginal fistula.12
Nerve Injury
Three types of nerve injury may occur during incontinence surgery. The first is a result of intraoperative positioning. The most common nerve injured is the common peroneal nerve; injury is caused by direct compression of the nerve against the leg support while the patient is in the dorsal lithotomy position. A femoral nerve palsy may result from hyperflexion of the hip wherein the thigh is brought too cephalad and in a prolonged position may cause postoperative weakness of the thigh extensor muscles. A second type of nerve injury involves direct trauma to the nerve during surgery. Intraoperative events may pose injurious risk to the ilioinguinal nerve as it travels in the vicinity of suprapubic transverse incisions, needles, slings, and trocar passages. Geis and Dietl first reported ilioinguinal nerve entrapment by TVT.42 Local anesthetic injections along the nerve can aid in diagnosis and treatment without the need for TVT removal. Patients present with lower abdominal wall pain radiating into the groin, labia majora, or upper inner thigh. Certainly, the obturator nerve is at risk in placement of midurethral synthetic slings (e.g., TVT, TOT).21,43,44 A third type of nerve injury involves disruption of the innervation of the urethra and surrounding structures. Some evidence suggests that vaginal dissection may affect this innervation. Zivkovic and colleagues45 showed that vaginal dissection, especially during endoscopic bladder neck suspension, can worsen preexisting perineal neuropathy in patients with SUI. Vaginal innervation seems to be concentrated on the anterior vagina46 and may be affected by anti-incontinence operations. Modification of innervation of the anterior vaginal wall could account for postoperative discomfort with intercourse.47 It is unknown whether alterations in neuroanatomy are responsible for some cases of sexual dysfunction after anti-incontinence procedures. Mazouni and associates48 reported that 25.6% of patients who were monitored prospectively with questionnaires had some deterioration of sexual function after TVT placement. Glavind and Tetsche49 found that 23% of previously sexually active women reported improved sexual life. Maaita and colleagues50 reported no change in sexual function after TVT procedures. Only prospective studies using validated symptom questionnaires will delineate the impact of TVT and incontinence treatment on sexual functioning.
EARLY POSTOPERATIVE COMPLICATIONS
Voiding Dysfunction
Immediate postoperative urinary retention is the most common type of voiding dysfunction encountered after any anti-incontinence procedure (Table 49-2). Kelly and colleagues51 reported acute urinary retention requiring catheterization in 41% of 114 women who underwent modified Pereyra bladder neck suspension. Twenty-seven percent of women undergoing MMK cystourethropexy had postoperative urinary retention, according to Parnell and associates.52 Yalcin and coworkers53 noted that 3.2% of patients had temporary voiding dysfunction after TVT. In most of these patients, urinary retention (complete or partial) is transient, secondary to postoperative edema of the bladder neck and urethra, and resolves with catheter drainage. The estimated probability of temporary urinary retention lasting longer than 4 weeks is 5% for retropubic and transvaginal suspensions and 8% for sling procedures.54
Irritative or storage symptoms, including frequency, urgency, and urge incontinence, are also common after incontinence surgery. Usually, this is not a problem in the immediate postoperative period, if the patient is well prepared. It is important to be sure that the patient is emptying well; if not, intermittent catheterization should be considered. In the patient who is emptying well, a trial of anticholinergic drugs can be used. Sometimes, however, it is only time that will cure this problem. Postoperative voiding symptoms are likely to persist for up to 4 weeks. Patients with preoperative urgency are more likely to have continued urgency postoperatively (13% to 68% for pubovaginal slings and 22% to 79% for retropubic suspensions) than those with no preoperative urgency (3% to 45% and 0% to 33%, respectively).54
Infection
Urinary tract infections (UTIs) in the immediate postoperative period are uncommon, provided that preoperative urine cultures are negative and perioperative antibiotics are employed. If patients present with acute symptoms of a UTI, including increased irritative symptoms, during the first few months after surgery, urine culture should be obtained, and if it is positive the patient should be treated with appropriate antibiotics. Debodinance and colleagues55 found an incidence of UTI of 4.5% in a series of 800 patients having anti-incontinence procedures, of whom 281 had TVT. UTI rates are usually related to length of catheterization. Rarely, a patient develops recurrent postoperative UTI despite complete emptying of the bladder. In these cases, cystoscopy should be performed to rule out suture or sling erosion (see later discussion). However, in our experience, there are a small number of patients who develop recurrent UTI after incontinence surgery with no obvious cause.
The incidence of wound infections varies from 2% to 16%.56–58 The wound infection rate after TVT was 0.4% in a single series.9 Predisposing factors are no different than those for any other surgery and include obesity, diabetes, immunocompromise, and reoperation. The vagina is a contaminated space; therefore, we routinely cleanse the vagina with a Hibiclens or povidone sponge before the surgical preparation to minimize infection. Perioperative antibiotics are important in the prevention of infection. We routinely use ampicillin and gentamicin perioperatively for the prevention of infection. An oral cephalosporin or quinolone is used for 7 days postoperatively.
Pelvic infections and abscess are rare occurrences. Appropriate management should include broad-spectrum antibiotics and usually surgical drainage (vaginal or abdominal) depending on size. Neuman managed two infected hematomas after TVT with ultrasound-guided aspiration and antibiotics, avoiding open surgery and removal of TVT material.59 One case of necrotizing fasciitis with massive debridement after TVT has been reported, but it is questionable whether this was primarily related to the TVT or whether seeding or contamination of incision sites from a distal infected extremity occurred.60
DELAYED OR LATE POSTOPERATIVE COMPLICATIONS
Pain
After needle suspension, some patients experience a chronic suprapubic pain that may radiate down the medial aspect of the inner thigh or cause a pulling sensation. This is thought to occur because of entrapment of the sensory branches of the ilioinguinal nerve or the genital branches of the genitofemoral nerve as the suspension sutures are tied to the rectus fascia.61 This complication was noted in as many as 8% to 16% of patients in early series.62 The passage of needles and trocars closer to the midline and adjacent to the pubic bone should reduce the risk of this complications. Hilton and associates63 reported two cases of chronic vaginal and pelvic pain of unknown etiology in patients who were eventually found to have eroded intraurethral/bladder neck TVT. We also have seen patients with pain out of proportion to physical examination findings who were subsequently found to have urethrally eroded TVT.
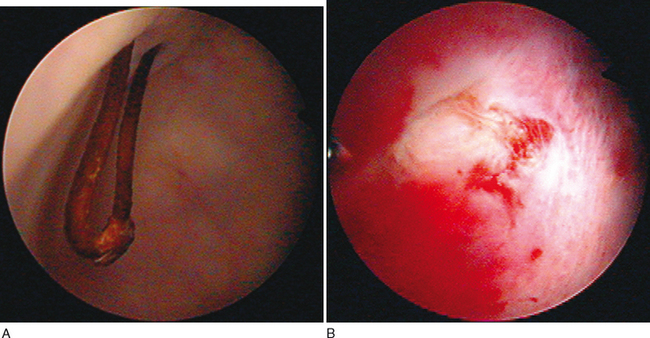
Suture Erosion
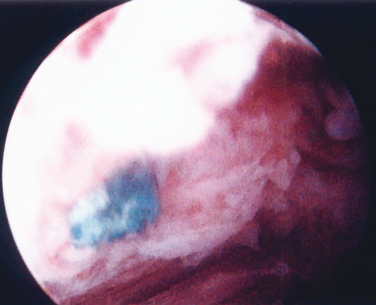
Rarely, a patient presents with postoperative irritative symptoms and/or recurrent UTI secondary to a permanent suture that has “eroded” into the bladder (Fig. 49-2A). These sutures, or slings in the case of TVT,64 may act as a nidus for stone formation. It is more likely that this phenomenon occurs due to unrecognized placement of sutures into or through the bladder rather than an actual erosion. Most of the time, suture erosion can be treated endoscopically by cutting the suture, which often retracts through the bladder wall, or by using the holmium: yttriumaluminum-garnet (YAG) laser to ablate the suture and/or stone (see Fig. 49-2B).65
Infection and Erosion of Sling Material
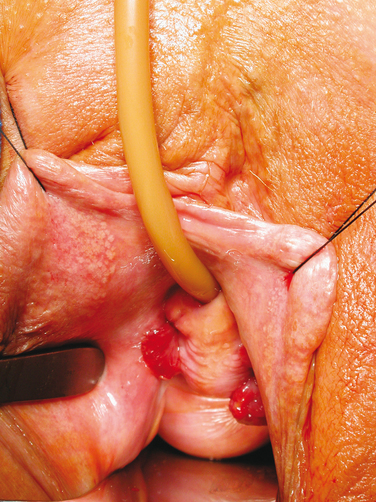
Vaginal Extrusion
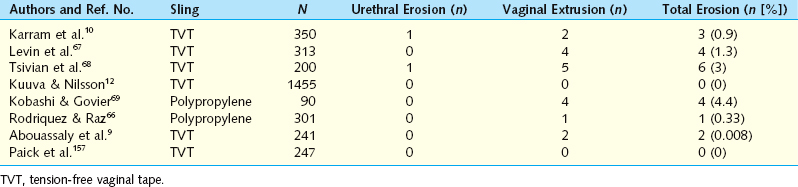
The exposure of synthetic material uncovered by vaginal epithelium is more properly termed vaginal extrusion rather than vaginal erosion (Fig. 49-3; Table 49-3). Vaginal extrusion is uncommon. Possible reasons for this complication are subclinical infection, inadequate vaginal incision closure, impaired wound healing, and tape/sling rejection. For the TVT, local vaginal flaps around the extruded site can be created and advanced over the exposed tape. Alternatively, the exposed tape can be excised, with vaginal flaps used to close the defect. A small area may be observed and treated with local estrogen cream, allowing re-epithelialization. Vaginal extrusion rates for TVT and polypropylene slings have varied from 0% to 4.4%.10,12,66–69 Other materials, such as polyester multifilament (Mersilene), silicone, polytetrafluoroethylene (Gore-Tex, Teflon), or even multifilament polypropylene, have higher extrusion and infection rates.70 Pore size less than 10 μ m increases the risk of infection, because bacteria may enter the interstices but macrophages and neutrophils larger than 10 μ m cannot penetrate the pores of the mesh to fight infection. Monofilament polypropylene also may have better incorporation into surrounding tissues, because its pores allow passage of fibrocytes.
Infected type I mesh can usually be removed partially or in the segment involved. TVT is a macroporous type I mesh with pores larger than 75 μ m that allow bacteria, macrophages, and fibroblasts to enter the pores.71 An exception is when type I mesh has been anchored to tissue using permanent multifilament sutures that harbor infection. Types II and III mesh usually have to be removed entirely if they become infected. Type III mesh has microporous portions that allow sequestration of bacteria but not macrophages.72 TVT extrusion in the lateral vaginal fornices may result from improper placement of the sling during the TOT technique when the vaginal epithelium is inadvertently punctured with the trocar.
Urethral Erosions
Urethral erosion is quite uncommon. Leach and coworkers54 reported an erosion rate of 0.003% with autologous slings and 0.02% with synthetic slings. It most likely results from dissection too close to the urethra with thinning and devascularization of tissues, placement of the sling between the urethra and periurethral fascia, excessive tension, or direct urethral injury including perforation that is missed. If the periurethral fascia is disrupted beneath the urethra, then it is best to repair the periurethral fascia before placement of the suburethral sling. In the use of trocars, more caution should be used when passing trocars toward the urethra rather than away, because the surgeon usually has less precise control of the distal aspect of the trocar. Small (seemingly inconsequential) movements of the proximal aspect of trocars translate to much larger ranges of movement in the distal trocar. Finger guidance is very helpful when trocars are passed toward the urethra.
A high index of suspicion must be maintained, because the presentation of urethral erosion can be quite delayed and of variable symptomatology. Patients may have dysuria, irritative or obstructive symptoms, recurrent incontinence, pain, vaginal discharge, UTIs, hematuria, pyuria, or other symptoms and signs. Slings can be removed endoscopically by cutting each end of the eroded material.73,74 We prefer an open approach via transvaginal suburethral incision in which all eroded material is removed from the urethral wall. Repair of the urethra with absorbable suture should be performed, and a catheter should be left in place for 5 to 10 days, depending of the extent of the injury. In severe cases, local flaps may be necessary to cover the repair and prevent fistulization.
For intravesical synthetic sling erosions or placements, the holmium: YAG laser may ablate the intravesical portion of the sling. For more complicated cases, an open retropubic approach may be needed for complete excision (Fig. 49-4).
Osteitis Pubis and Osteomyelitis
Osteitis pubis is a noninfectious inflammation of the periosteum overlying the symphysis pubis that usually occurs 1 to 8 weeks after the inciting event. It manifests with symptoms and signs of suprapubic pain and tenderness, adductor spasm, elevated erythrocyte sedimentation rate, and a “waddling” gait. It has been historically associated with the MMK cystourethropexy, and its incidence ranges from 1% to 10%, depending on the series.52,75,76
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree