the degree of dialysis time prior to transplant has been associated with poorer graft survival following transplant.9
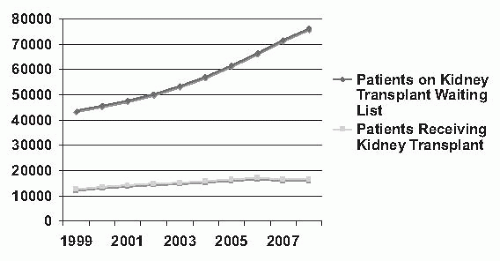 FIGURE 82.1 Counts of patients on the renal transplant waiting list and counts of renal transplants by year in the United States from 1999 to 2008. (From Scientific Registry of Transplant Recipients 2009 Annual Data Report, Ann Arbor MI, Tables 5.1a, 5.1b, 5.4, 5.4d, with permission.) |
TABLE 82.1 Factors Influencing the Outcome of Renal Transplantation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
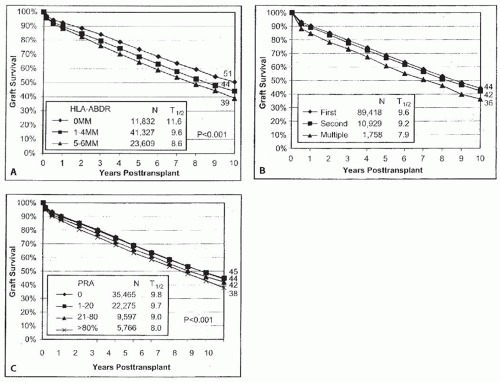
TABLE 82.2 Actual Transplant Half-Life for Transplants Performed in 1997, and Projected Transplant Half-Life for Transplants Performed in 20049 | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
TABLE 82.3 Contraindications to Transplantation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
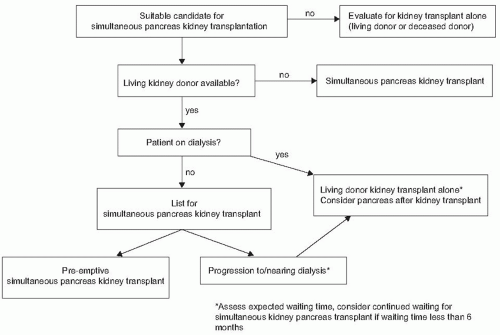
the long-term graft survival is unknown and the risks of immunosuppression and HLA sensitization must be weighed against the benefits of normalization of blood glucose.
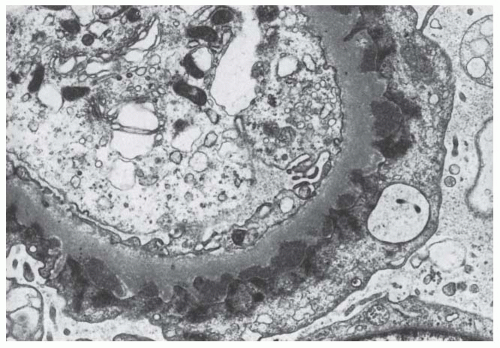
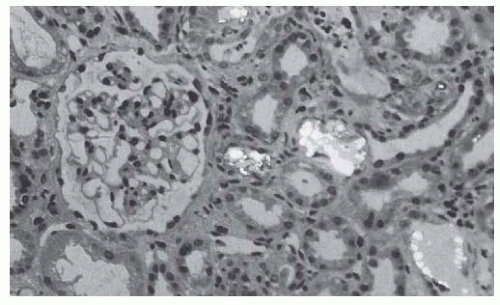 FIGURE 82.6 A renal biopsy specimen from a transplanted kidney showing calcium oxalate deposition in a patient with primary hyperoxaluria and a recurrence of oxalosis. |
less invasive treatment strategy in the future.43 Occasionally, patients with severe liver cysts will require combined liver-kidney transplantation, primarily due to symptoms related to cyst volume and the impact on nutritional status.44 Screening for cerebral aneurysms prior to transplant is generally directed toward those with a family history or with new onset headaches.45
TABLE 82.4 Recurrent Disease in Renal Allografts | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
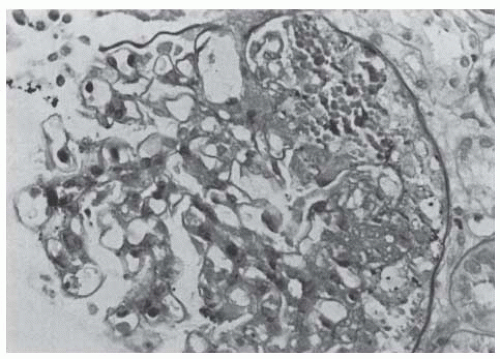 FIGURE 82.7 A renal biopsy specimen of a transplanted kidney showing recurrence of focal segmental glomerulosclerosis. (Periodic acid-Schiff stain, magnification ×250.) |
column or plasma exchange can reduce protein excretion in patients with recurrent FSGS in the transplant. More prolonged remissions have been achieved using plasma exchange that is initiated promptly after the onset of proteinuria or the combination of plasma exchange and cyclophosphamide. These prolonged beneficial results have also been reported in children treated with plasma exchange and cyclophosphamide.52
TABLE 82.5 Pretransplantation Recipient Medical Evaluation | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
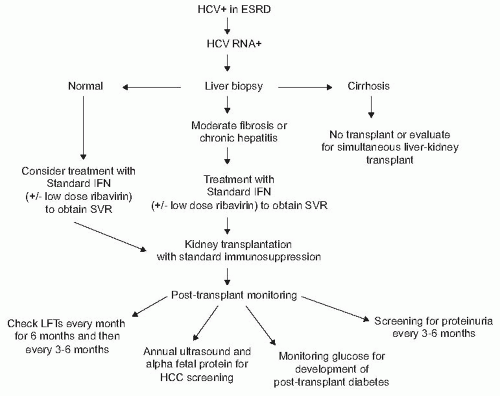
after transplantation in the setting of chronic immunosuppression, the survival benefit of transplantation over dialysis outweighs this risk. Transplant candidates who are HCV+ with detectable RNA and no clinical stigmata of cirrhosis should undergo liver biopsy to determine histologically the degree of underlying liver disease. In those with cirrhosis, combined liver-kidney transplantation should be considered (Fig. 82.9). In those without cirrhosis, antiviral therapy should be considered to minimize the risk of developing posttransplant complications.92 Goals of therapy are not only to avoid progressive liver disease, but also to avoid the extrahepatic complications such as the development of new onset diabetes after transplantation (NODAT) or glomerulonephritis that may occur in HCV infected renal transplant recipients.93 A 48-week course of pegylated interferon (IFN)-α and ribavirin is often used in non-CKD populations. Unfortunately, in the setting of CKD, rapid accumulation of ribavirin can occur, which can lead to significant hemolysis. In the setting of CKD, pegylated IFN-α is associated with a high rate of adverse effects that lead to discontinuation of this therapy with no demonstrable benefit in sustained viral response (SVR) over nonpegylated IFN-α. Therefore, in patients on dialysis, monotherapy with nonpegylated IFN-α for 24 to 48 weeks is suggested as first-line therapy, with viral response rates as high as 70% to 80%, with the average SVR of 30% to 40%.
has published consensus guidelines that attempt to take into consideration a number of more common clinical circumstances, but these must continue to be reviewed in the context of emerging data.80 Oncology referral and discussion of expected disease-free survival is an important part of the evaluation process for those with a history of malignancy.
contraindications to transplant include a positive CDC cross-match or T-cell FCXM.105 A number of transplant centers in the United States have forgone the CDC method in favor of FCXM and SAB analysis, tests that offer improved sensitivity at the likely expense of decreased specificity. For example, although a positive CDC cross-match has remained an absolute contraindication to transplant, the clinical implications of a weak FCXM or low level antibodies detected by SAB are less clear and are currently a matter of intense clinical research. Thus, the evolution of cross-match techniques has resulted in increasing protection against early AMR at the expense of potentially withholding the transplant in patients with clinically irrelevant antibodies detected by sensitive assays.
donate a kidney for purely altruistic reasons, provides an opportunity to benefit individuals who may have an incompatible donor or individuals without a living donor option.117 Paired exchange programs have been developed to identify two potential donors who wish to donate to a family or friend but are unable to due to blood group incompatibility or a positive cross-match. Two such donors and their prospective recipients are then paired, with donor A donating to recipient B and donor B donating to recipient A. When an altruistic donor is introduced to paired exchange programs, it may result in significant opportunity for transplantation of a number of incompatible pairs.118,119 Another circumstance is the matched donor in which a prospective recipient pays a monthly fee to a coordinating site, which presumably has access to a list of potential parties interested in donating their kidney. In the United States, assurances required from these donor/recipient circumstances must include the lack of monetary benefit for the donor (altruism). The U.S. Organ Transplantation Act of 1984 (HR5580, Title II) makes it a federal crime to engage in organ sale and commerce. Other countries have eliminated the waiting list with the use of monetary incentives for living unrelated donation, a topic that continues to be debated worldwide.120,121
TABLE 82.6 Suggested Evaluation Process for Potential Living Donors | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 82.7 Exclusion Criteria for Living Kidney Donors | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
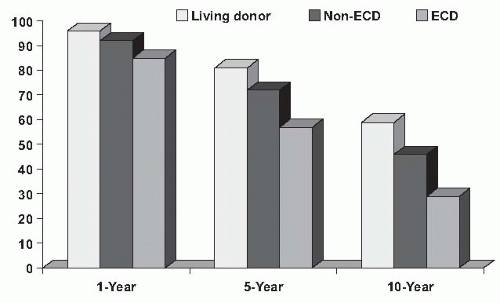
that increased the risk for graft failure by 70% compared with an SCD kidney and include donors over the age of 60, or donors between the ages of 50 to 59 with two of three additional criteria: (1) cerebrovascular accident as a cause of death, (2) prior diagnosis of hypertension, or (3) terminal serum creatinine greater than 1.5 mg per day. The rationale for making the distinction between SCD and ECD was to allocate kidneys efficiently to those in greatest need (those at greatest risk for mortality while on dialysis).122 The survival benefit of ECD transplant over dialysis is present across all candidates, but in particular is of benefit to those with diabetes over the age of 40 or who are in regions with waiting times for a transplant of > 1,350 days.5
TABLE 82.8 Medical Evaluation of the Potential Deceased Donor | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
must be given to retraction of the kidney during its removal to avoid traction injury of the renal artery and dissection in the hilum of the kidney, particularly between the ureter and the renal artery, which should be avoided to prevent damage to the ureteric blood supply. Furthermore, in removing the ureter down to the brim of the pelvis, care should be taken to leave an adequate amount of periureteric tissue. A living donor nephrectomy for transplantation can also be performed by laparoscopic approach.127 This approach results in less postoperative surgical pain, a shorter hospital stay, and a quicker recovery than the standard open donor nephrectomy (Table 82.9). The laparoscopic techniques have been rapidly adopted worldwide; an analysis from Australia/ New Zealand transplant centers upon the introduction of the laparoscopic technique in 1997 through 2004 demonstrates comparable rates of technical failure, delayed graft function, and graft survival to an open nephrectomy, with a conversion rate to open procedures of 6%.128 This conversion rate is much higher than that reported for experienced centers of 1%.129
TABLE 82.9 Advantages and Disadvantages of Laparoscopic Nephrectomy | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
of kidneys from less traditional donors has been on the rise, including ECD and DCD, both of which are associated with significantly higher rates of DGF.134,135 As a result, a number of recent clinical trials have studied preservation methods in an attempt to demonstrate improved rates of DGF in deceased donor kidney transplants.
TABLE 82.10 Contents of Commonly Used Cold Preservation Solutions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
catheter is generally left in these patients for 3 to 4 days because of high urine outflow rates that occur during this time in order to prevent overdistension of the bladder. This is particularly important in diabetic patients who frequently have neurogenic bladders and can have extremely large bladder volumes before they develop an urge to micturate. Catheters should also be carefully monitored for obstruction and irrigated under sterile conditions if occluded by a clot.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree