Hypertension due to Primary Aldosteronism and Related Disorders
Emmanuel L. Bravo
Muhammed A. Rafey
Surafel F. Gebreselassie
INTRODUCTION
Distinct hypertensive syndromes related clearly to mineralocorticoid overproduction are increasingly being recognized. Primary aldosteronism (PA), first described by Conn in 1955,1 is characterized by hypertension, hypokalemia, suppressed plasma renin activity (PRA), and increased aldosterone production. Reversal of the clinical manifestations by the surgical removal of a right adrenal adenoma established the relationship among aldosterone overproduction, hypokalemia, and hypertension for the first time.
Three heritable forms of aldosteronism are known today. FH-1 is a glucocorticoid-remediable form of aldosteronism (GRA). Two glucocorticoid-resistant forms have been described: FH-II, the familial occurrence of aldosterone producing adenoma (APA), or bilateral adrenal hyperplasia and FH-III, the familial occurrence of massive bilateral adrenal hyperplasia.
Excessive production of deoxycorticosterone (DOC) results from (1) disorders of steroid production (congenital adrenal hyperplasia), (2) generalized glucocorticoid resistance, and (3) DOC-producing adrenocortical tumors.
A group of disorders simulating PA but with a low production of aldosterone or other mineralocorticoids has also been described. These disorders occur as a result of increased activation of mineralocorticoid receptors (MR). The list includes (1) apparent mineralocorticoid excess (AME), (2) cortisol excess, (3) gain-of-function mutation of ENaC of distal renal tubules (Liddle syndrome), and (4) hypertension exacerbated by pregnancy due to a mutation of mineralocorticoid receptors.
PRIMARY ALDOSTERONISM
PA is the most common and important cause of secondary hypertension. The urgency in identifying PA early and treating it is heightened by data from studies demonstrating that persistent elevation of aldosterone levels can result in end organ damage. In addition, findings from animal studies indicate that cardiovascular and renal injury occur independently of blood pressure levels.2 Cross-sectional studies in humans demonstrate early subclinical end organ damage that includes an increase in carotid intima media thickness and worsening in pulse wave velocity, both gold standard measures of arterial wall stiffness. Further, these studies have shown reduced endothelial function when compared to matched controls with essential hypertension. Several studies have also demonstrated clear evidence of increased left ventricular hypertrophy, diastolic dysfunction, myocardial fibrosis, and albumin excretion rate when compared to those with primary hypertension.3,4,5,6,7,8,9,10 It appears that these deleterious end organ effects could potentially be ameliorated with early and appropriate medical and surgical intervention.11
Pathophysiology of Mineralocorticoid-Induced Hypertension
Aldosterone, a major mineralocorticoid hormone, has potent effects on unidirectional transepithelial sodium transport. Inappropriately elevated aldosterone levels drive sodium and water retention, which increases circulatory volume and cardiac output; the latter, in turn, is reflexively normalized by vasoconstriction, resulting in hypertension. It is reasoned that the increase in total peripheral resistance to maintain the elevated arterial blood pressure occurs later, following vascular autoregulation.12 However, hypervolemia is not a universal finding in patients with PA.13 Many patients have either low or normal intravascular volume and there is no correlation between arterial blood pressure and plasma or total blood volume in either men or women with untreated PA. The potent effects of aldosterone on salt and water retention reasonably suggest that hypervolemia might have had a role in initiating the pressure rise and then may have been superseded or even partially reversed by other mechanisms. One could speculate that a secondary rise of resistance after an initial increase in cardiac output would establish new levels of equilibrium. The accumulated evidence favors the conclusion that aldosterone, by producing functional changes in the arterial wall, is responsible for the
initial vasoconstrictive response and the sustained and progressive hypertensive state that follows. Most experimental evidence from intact animal studies suggests that mineralocorticoids both increase membrane permeability to sodium and elevate intracellular sodium concentration, which in turn decreases calcium efflux.14 By partially depolarizing the muscle cell membrane, the abnormalities of cation turnover lead to vasoconstriction and elevated vascular resistance. Such changes also increase metabolic activity and provide an early signal for vascular smooth hypertrophy, which when combined with rising blood pressure, could lead to thickening of the media and so raise the wall-to-lumen ratio. This structural adaptation implying enhanced reactivity could be crucial for both potentiating and maintaining the hypertensive process.15 The study suggests that an increase in systemic vascular resistance leading to hypertension could occur independent of changes in intravascular volume.
initial vasoconstrictive response and the sustained and progressive hypertensive state that follows. Most experimental evidence from intact animal studies suggests that mineralocorticoids both increase membrane permeability to sodium and elevate intracellular sodium concentration, which in turn decreases calcium efflux.14 By partially depolarizing the muscle cell membrane, the abnormalities of cation turnover lead to vasoconstriction and elevated vascular resistance. Such changes also increase metabolic activity and provide an early signal for vascular smooth hypertrophy, which when combined with rising blood pressure, could lead to thickening of the media and so raise the wall-to-lumen ratio. This structural adaptation implying enhanced reactivity could be crucial for both potentiating and maintaining the hypertensive process.15 The study suggests that an increase in systemic vascular resistance leading to hypertension could occur independent of changes in intravascular volume.
Prevalence
The prevalence of PA has remained debatable as studies have been fraught with several limitations, which include bias in patient selection and reliance on tests that are not regarded as confirmatory for the diagnosis of PA.16 A large prospective clinical trial, the PAPY (PA Prevalence in Hypertensives) Study, demonstrated that PA involves at least 11.2% of consecutive patients with newly diagnosed hypertension. Although the patients underwent a thorough workup that allowed the investigators to definitively establish the presence or absence of PA, this may still be an overestimation of PA prevalence in patients with hypertension as study participants were enrolled from specialized hypertension clinics.17 Gordon and coworkers18 reported the incidence of 12% of PA in 199 patients referred to their clinic for hypertension. The clue that a much lower prevalence of PA exists in patients with hypertension is provided by another study by Douma et al.19 that evaluated patients with resistant hypertension, which showed that 11.3% of patients in this group had PA based on the confirmatory salt suppression test. Based on the prevalence of PA in patients with resistant hypertension in the general hypertensive population and the lower prevalence of PA in milder forms of hypertension, we could assume that the prevalence of PA in the general unselected hypertensive population is much lower than currently thought. Therefore, the actual prevalence of PA among unselected hypertensives is still unknown, but can be estimated from the data of Mosso et al.,20 considering the relative proportion of the different grades of hypertension in the general population is around 4%.
Case Detection of Primary Aldosteronism
A high index of suspicion for PA must be entertained in patients who develop (1) spontaneous or unprovoked hypokalemia; (2) severe hypokalemia (<3 mEq per liter), which does not normalize even with potassium replacement or addition of potassium-sparing diuretics; (3) potassium levels that do not normalize even after discontinuation of diuretics for 4 weeks; (4) resistant hypertension with no other evidence of secondary cause; (5) hypertension with adrenal adenoma; (6) and those who have a family history of PA, early onset hypertension, or cerebrovascular accident at a young age (<40 years).
Serum Potassium
Traditionally, spontaneous hypokalemia (serum K <3.5 mEq per liter) has been regarded as the most effective screening test to diagnosis PA. But data from our studies and others have shown that a substantial number of patients with PA do not present with hypokalemia.13 The reported prevalence of normokalemia in PA is variable. Conn reported a 7.6% prevalence of normokalemia in his series of 145 patients.21 In the PAPY study, only 9.6% of patients with PA were found to have spontaneous hypokalemia.17 In a study by Douma et al.,19 45.6% of patients with PA demonstrated hypokalemia. Others have shown a normal serum potassium concentration in 7% to 38% of reported cases.21,22 In addition, 10% to 12% of patients with proven adrenocortical tumors may not develop hypokalemia during short-term salt loading. A normal serum potassium does not rule out PA; however, spontaneous hypokalemia associated with renal potassium wasting (UkV ≥ 30 mEq per liter) has a high sensitivity and specificity in the diagnosis of PA.
Plasma Aldosterone Concentration:Plasma Renin Activity Ratio (ARR)
The plasma aldosterone concentration (PAC):plasma renin activity (PRA) ratio is considered the best screening test for PA. This technique is highly sensitive but has a high false-positive rate (about 30% to 50%) because PRA, the denominator, can be very low (as low as 0.1 ng per milliliter per hour) in some laboratories. Accordingly, a minimum PRA of 0.65 ng per milliliter per hour is recommended in calculating the ratio.23 In published studies, the screening cutoff values vary from 7.2 to 100 ng per deciliter per nanogram per milliliter per hour; consequently, there is wide variation in the sensitivity (64% to 100%) and specificity (87% to 100%) of the test.24 Reported ratios are all laboratory dependent. In a large, multicenter, prospective trial the accuracy of ARR for pinpointing patients with aldosterone-producing adenomas (APA) was close to 80%.25 In addition, study results demonstrated a highly significant within-patient correlation (r=0.69; P< .0001) and reproducibility (coefficient of determination: 0.47). Better diagnostic accuracy is obtained if the absolute PAC is included as a second criterion in combination with the ARR. In a retrospective study,26 the combination of a PAC:PRA ratio >30 and a PAC value >20 ng per deciliter had a sensitivity of 90% and a specificity of 91% for APA.26 At the Mayo Clinic, a PAC:PRA ratio of ≥20 and a PAC >15 ng per deciliter were found in more than 90% of patients with surgically confirmed APA.27
Several factors such as time of day, diet, posture, method of blood collection, and plasma potassium level may affect
ARR sensitivity and specificity. Other factors that may affect ARR sensitivity and specificity include age, gender, race, diabetes mellitus, and use of oral contraceptive agents. Most elderly and diabetic patients have low levels of PRA. About 40% of essential hypertensive patients have low PRA and about 27% of untreated patients with PA may have nonsuppressed PRA.13 Pizzolo and coworkers28 reported that oral contraceptive administration may increase ARR, contributing to the diagnostic inaccuracy in women. Only 36.9% of women with positive ARR had confirmed PA by intravenously administered salt.
ARR sensitivity and specificity. Other factors that may affect ARR sensitivity and specificity include age, gender, race, diabetes mellitus, and use of oral contraceptive agents. Most elderly and diabetic patients have low levels of PRA. About 40% of essential hypertensive patients have low PRA and about 27% of untreated patients with PA may have nonsuppressed PRA.13 Pizzolo and coworkers28 reported that oral contraceptive administration may increase ARR, contributing to the diagnostic inaccuracy in women. Only 36.9% of women with positive ARR had confirmed PA by intravenously administered salt.
Blood samples are best obtained in the morning in an ambulatory seated patient. Ideally, all antihypertensive medications should be discontinued 2 to 3 weeks before ARR testing, but in many patients, this is not feasible. Although some drugs may alter the accuracy of the PAC:PRA ratio, they are not usually an issue in patients with PA. Thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers can actually improve the diagnostic discriminatory power of the PAC:PRA ratio, whereas beta-adrenergic blockers and central alpha-2 agonists suppress PRA and may give false-positive results,29 especially if the absolute PAC cutoff is not used. If blood pressure control is an issue, alpha-1-adrenoreceptor blockers and/or a nondihydropyridine calcium channel blocker (such as verapamil) may be used. Furthermore, specific aldosterone antagonists may stimulate plasma renin activity, giving rise to false-negative results.
Confirmatory Test(s)
The diagnosis of PA can often be established with relative ease. In the hypertensive patient receiving no treatment who demonstrates significant hypokalemia (<3 mEq per liter) with renal potassium wasting (24-hour urinary potassium >30 mEq), PRA below 1 ng per milliliter per hour, and elevated plasma or urinary aldosterone values, the diagnosis of PA is unequivocal and may not undergo salt suppression testing. Often, however, the diagnosis is not obvious because of equivocal values. In such cases, multiple measurements are needed during salt loading. Cleveland Clinic patients ingest a normal diet with additional salt added (1 tsp table salt) to food each day for 5 consecutive days. On the 5th day of increased dietary salt, 24-hour urine is collected for sodium, potassium, aldosterone, and creatinine. On the morning of the 6th day, blood is drawn for basic metabolic panel, aldosterone, and renin activity. A 24-hour urinary aldosterone ≥14 µg per day (mean +2 standard deviations above values obtained in essential hypertensives) is definitive evidence of a nonsuppressible aldosterone production as long as the 24-hour urinary sodium is ≥200 mEq. The development of hypokalemia (serum K <3.5 mEq per liter) with renal potassium wasting (UkV ≥30 mEq per liter) provides additional evidence of inappropriate aldosterone production. An aldosterone excretion greater than 14 µg per 24 hours following salt loading distinguishes most patients with PA from those with primary hypertension; only 7% of patients with PA have aldosterone excretion values that fall within the range obtained in primary hypertension.13
Because most classes of antihypertensive medications affect plasma aldosterone levels and PRA values, antihypertensive treatment should be modified 4 to 6 weeks before the salt loading test. Long-acting calcium channel blockers and the alpha-adrenoreceptor antagonist doxazosin may be used during this period to control blood pressure when required.
Other aldosterone suppression testing has been described.30 These include intravenous sodium chloride loading, captopril stimulation, or fludrocortisone suppression with the measurement of PAC. The captopril stimulation test operates on the principle that ACE inhibition is without effect when PRA is suppressed and plasma aldosterone is elevated while PRA increases and plasma aldosterone decreases in patients with secondary aldosteronism. However, the specificity of the test is markedly compromised by the large number of hypertensive patients with suppressed PRA (i.e., in low renin essential hypertension, the elderly, diabetics, and African Americans).
Differentiation of Subtypes
The two major causes of PA are bilateral adrenal hyperplasia (BAH), accounting for 65% to 70% of PA patients and aldosterone-producing adenoma (APA) accounting for 30% to 35% of PA. Unilateral hyperplasia and familial hyperaldosteronism accounts for 3% to 4% of patients with PA.31 It is important to differentiate those with APA because this is a potentially curable condition with surgical intervention.
Biochemical. Adenomas are likely to be present in a patient with spontaneous hypokalemia (<3 mEq per liter) and plasma 18-hydroxycorticosterone levels greater than 100 ng per deciliter. In addition, hyperaldosteronism resulting from a unilateral adrenal abnormality is exquisitely responsive to adrenocorticotropic hormone (ACTH) and not to angiotensin II infusions.32 A plasma 18-hydroxycorticosterone level < 100 ng per deciliter or a postural increase in plasma aldosterone, or both, are usually associated with adrenal hyperplasia but do not completely rule out the presence of an adenoma.13 However, a postural decrease in PAC has a high positive predictive value for the diagnosis of APA because a postural decrease in PAC does not occur in hyperplasia.
Localization of Aldosterone Hypersecreting Adrenal Gland.
Adrenal Computed Tomography Scan. All patients diagnosed with PA should undergo an adrenal computed tomography (CT) scan as the initial study in subtype differentiation.33 A high resolution CT scan with contrast with fine cuts (2.5 to 3 mm) is the imaging technique that displays the best sensitivity and specificity; it is generally more available and less costly than magnetic resonance imaging (MRI). Adrenal
cortical adenomas typically have low X-ray attenuation (≤10 HU) in the noncontrast enhanced CT examination (Fig. 43.1). By adding a CT examination approximately 15 minutes after the start of the intravenous contrast enhancement, the washout rate of the iodine contrast medium from the tumor is typically faster (40%) in benign cortical adenomas compared to nonadenomas. Adenomas that are 1.5 cm or larger in diameter can accurately be detected with this procedure. Only 60% of adenomas that measure 1.0 to 1.4 cm are detected, whereas nodules that are less than 1 cm in size are more likely to be missed by a CT scan. Reported sensitivity rates of localizing adenomas by CT scans are between 75% and 80%.34
cortical adenomas typically have low X-ray attenuation (≤10 HU) in the noncontrast enhanced CT examination (Fig. 43.1). By adding a CT examination approximately 15 minutes after the start of the intravenous contrast enhancement, the washout rate of the iodine contrast medium from the tumor is typically faster (40%) in benign cortical adenomas compared to nonadenomas. Adenomas that are 1.5 cm or larger in diameter can accurately be detected with this procedure. Only 60% of adenomas that measure 1.0 to 1.4 cm are detected, whereas nodules that are less than 1 cm in size are more likely to be missed by a CT scan. Reported sensitivity rates of localizing adenomas by CT scans are between 75% and 80%.34
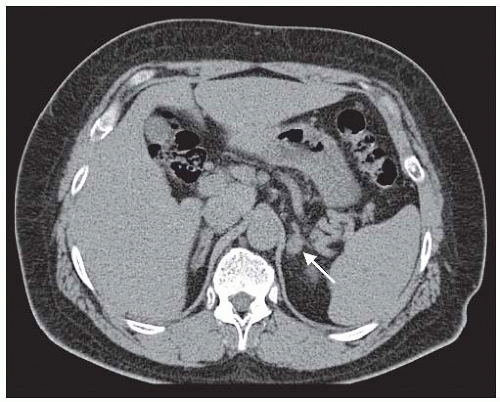 FIGURE 43.1 A 1.5 cm left adrenal adenoma (arrow) in the noncontrast enhanced computed tomography examination. The mass has an attenuation value <10 H and a calculated washout rate >50%. These are radiologic features consistent with an adenoma. Adrenal venous sampling (results shown in Table 43.1) provided evidence of a unilateral, left aldosterone-producing tumor. |
Adrenal Vein Sampling for Aldosterone. The Endocrine Society Guidelines33 recommend that all patients for whom treatment is practicable and desired should undergo adrenal vein sampling (AVS). There are exceptions to this recommendation. A patient <40 years of age with spontaneous hypokalemia, PAC 15 to 20 ng per deciliter, PRA <1 ng per milliliter, with a solitary adrenocortical macroadenoma (>1 cm) that is discrete, uniform, of low attenuation (HU ≤ 10), has a contrast washout >40%, and a morphologically normal contralateral adrenal gland needs no further evaluation and should be referred for surgery. Older patients with PA with CT findings demonstrating bilateral morphologically normal or abnormal glands or unilateral microadenoma should be sent for AVS (Fig. 43.2). There is debate as to whether older patients with PA with adrenal CT characteristics identical to the 40-year-old patient described previously should undergo AVS for subtype characterization.
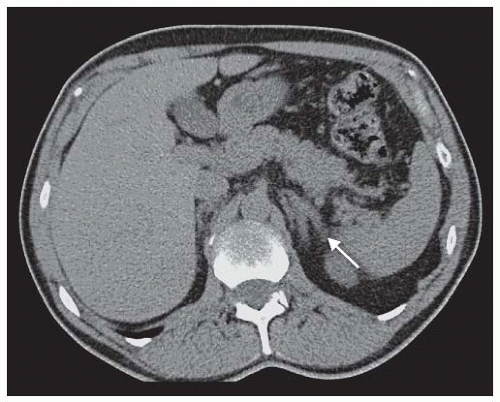 FIGURE 43.2 A noncontrast enhanced adrenal computed tomography scan of a patient with clinical characteristics suggestive of primary aldosteronism. There is marked thickening of both limbs of the left adrenal gland without discrete nodules. The right adrenal gland was reported as normal. Adrenal venous sampling (results shown in Table 43.2) showed bilateral secretion of aldosterone, indicating bilateral adrenal hyperplasia. |
AVS is technically difficult and requires skill and expertise. Even allowing for publication bias from large centers of excellence, success rates are variable ranging from 42%35 to 75%36 to 98%37 for successful bilateral cannulation. During AVS, continuous Cosyntropin is employed to increase adrenal blood flow and to augment aldosterone secretion from an APA. Blood samples are collected from the adrenal veins and the inferior vena cava (IVC) (peripheral sample) for the determination of cortisol and aldosterone concentrations. An adrenal vein:peripheral vein cortisol ratio of at least 3:1 indicates successful adrenal vein catheterization with 100% reproducibility. An aldosterone/cortisol (A/C) adrenal vein over an A/C contralateral adrenal vein of at least 4, plus an A/C contralateral adrenal vein/A/C IVC less than 1, indicates lateralization.38




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








