Risk
Approximately 40% of patients with diabetes develop clinically significant DN.
8,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26 A variety of clinical, epidemiologic, familial, and genetic factors predict the risk of the development of DN (
Table 58.1). Longer prepubertal duration of type 1 diabetes and prepubertal hyperglycemia increase the risk of postpubertal MA.
24 Older age at the time of diagnosis of type 1 and type 2 diabetes appears to increase the risk of DN,
19,
27,
28 but specifically in Pima Indians, it seems that the onset of type 2 diabetes prior to the age of 20 years confers a fivefold risk for ESRD in middle age as compared to onset after age 20.
22 A longer duration of diabetes is associated with an increased risk of DN,
29 but a majority of patients with diabetes (60%) do not ever develop clinically significant DN. Even slight elevations in body mass index (BMI) are associated with a higher risk of DN in patients with type 2 diabetes.
30 Very mild elevations of UAE (even within the normoalbuminuric range) predict a greater risk of development of DN.
30,
31,
32,
33,
34 In a 10-year prospective observational cohort, baseline UAE was 9 mg per 24 hours in those subjects who remained normoalbuminuric, but was 13 mg per 24 hours in those who ultimately developed MA or overt proteinuria.
31In the earliest stages of the changes to the kidney in diabetes there are both elevations in systemic blood pressure (BP) and glomerular hyperfiltration, which portend more serious injury. The earliest detectable marker of deranged BP regulation in type 1 diabetes is elevated nocturnal systolic BP. An early study demonstrated this correlation with systolic and diastolic BP obtained via 24-hour ambulatory BP monitoring (ABPM).
35 Elevation of nocturnal systolic BP was demonstrated in a prospective longitudinal cohort analysis of 75 adolescents and young adults with type 1 diabetes and normal urinary albumin excretion,
36 in which nocturnal systolic BP elevation by ABPM preceded and predicted the onset of MA. The risk of development of MA was 70% lower in those subjects with a normal nocturnal dipping status, even in those subjects with poor metabolic control (a known predictor of MA, see later text).
Elevated systemic BP at the time of diagnosis of diabetes is associated with the later development of DN, in both types 1 and 2 diabetes. In a cohort of patients with type 1 diabetes followed for 20 years after the onset of diabetes, those patients who were ultimately destined to develop DN (20 years later) had statistically significantly higher systolic and diastolic BP at the time of diagnosis of diabetes compared to those who never developed DN (mean BP 122/76 mm Hg in those subjects who did not develop MA, as compared to 128/80 mm Hg in those who did).
6 Further supporting the role of elevated systemic BP as a risk factor for the development of DN, Parving et al.
12 characterized the prevalence of hypertension (HTN) (defined, at the time, as > 160/95 mm Hg or on antihypertensive medications) in 982 subjects with type 1 diabetes attending a diabetes clinic, stratified according to albumin excretion. The presence of HTN strongly correlated with DN, such that HTN was present in 19%, 30%, and 65% of subjects with normo-, micro-, and overt proteinuria. Due to this high prevalence of hypertension at the time of diagnosis of type 2 diabetes, the presence of HTN is less predictive of the risk of developing DN in the future in type 2 rather than type 1 diabetes.
37,
38,
39Glomerular filtration rate (GFR) is higher at the onset of diabetes as compared to weight- and age-matched controls, both in types 1
40,
41,
42 and 2 diabetes.
43 In their study of 13 males with type 1 diabetes of short duration (mean duration 2.4 years), Christiansen et al.
42 demonstrated that iothalamate-GFR was increased in diabetes (144 vs. 113 mL per min), as were renal plasma flow and kidney volume (assessed by hippuran and ultrasound, respectively). Glomerular function was investigated in type 2 diabetic Pima Indians,
43 which demonstrated that iothalamate-GFR was 140 versus 122 mL per min in diabetic subjects as compared to nondiabetic controls, and was higher in subjects with impaired versus normal glucose tolerance (before the
onset of diabetes).
44 Although glomerular hyperfiltration is common at the time of diagnosis of diabetes, those patients destined to develop DN have, on average, higher GFR than those patients with diabetes who never develop DN.
45,
46 Despite the correlation between higher GFR at the onset of DM and the risk of developing DN, there is no absolute cut-off level of GFR above which DN develops with certainty in the future. Various mediators of hyperfiltration
47,
48,
49 have been postulated, including alterations in eicosanoids, nitric oxide, atrial natriuretic peptide, and transforming growth factor-beta. Treatment with continuously infused insulin for 2 years (via insulin pump) moderates the hyperfiltration in type 1 diabetes.
50Renal size is also increased in early diabetes.
51 Christiansen et al.
42 demonstrated that males with type 1 diabetes had mean renal volume of 278 mL per 1.73 m
2 versus 224 mL per 1.73 m
2 for nondiabetic control males, a significant increase of 24%. Treatment with insulin for 3 months was shown to reduce kidney size in newly diagnosed men with type 1 diabetes.
52 Interestingly, kidney size remains larger at ESRD in those patients with ESRD due to diabetes than from other causes.
53 In one study, renal length was estimated using ultrasonography, and mean right renal length was 9.9 versus 8.8 cm (DN vs. no DN); mean left renal length was 10.0 versus 9.1 cm.
African Americans, Asians, Polynesians, Maori, Native Americans, and Hispanic Americans with diabetes all have an increased risk of developing DN as compared to Caucasians with diabetes.
13,
54,
55,
56,
57,
58,
59,
60,
61 The overall incidence of diabetes-related ESRD in Jefferson County, Alabama, was 3.4 times higher in African Americans than in Caucasians
56; similarly, the incidence was 4.4 times higher among African Americans with ESRD reported to the Michigan Kidney Registry from 1974 to 1983.
13 In Mexican Americans studied in the Texas Kidney Health Program over the period 1978 to 1984, the incidence of diabetes-related ESRD was six times higher than in non-Hispanic whites.
59 The prevalence of DN (as estimated by a single dipstick assessment of MA) in a global cohort of type 2 diabetes was nearly 40% higher in Asians, and 30% higher in Hispanics, than in Caucasians.
54 In addition to certain groups having an increased risk of developing DN, it appears that some have an accelerated rate of decline of renal function once DN is established.
62In those families in which multiple members have diabetes, the presence of DN in one member predicts an increased risk of DN in other family members.
63,
64,
65,
66,
67,
68 An early report demonstrated that there was evidence of DN in 83% of the siblings of probands who had undergone renal transplantation for DN.
63 In this study, the presence of nephropathy in the proband was the only significant predictor of the presence of it in the sibling. These clinical observations have led to studies
69 to identify genetic markers that predict the development of DN. Candidate genes span many gene classes, and were recently summarized,
70 but include glucose transporter 2, kininogen, adiponectin, transforming growth factor-beta II and III, catalase, endothelial nitric oxide synthase, apolipoprotein E, tissue inhibitor of metalloproteinase 3,
71 and angiotensin-I converting enzyme.
72 Identification of genes involved in the pathogenesis of DN will likely help direct the development of novel agents to treat it.
Injury
Albuminuria (from MA to overt proteinuria) and loss of GFR represent a spectrum of pathologic diabetic injury to the kidneys. We review these forms of diabetic kidney injury in turn.
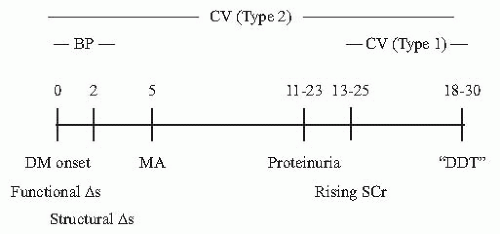
MA has traditionally been considered the hallmark of DN, and the earliest clinical feature of it. MA occurs in patients with either type 1 or type 2 diabetes. Approximately 10% to 20% of patients with type 1 diabetes develop MA after 5 to 15 years of diabetes.
11 It is important to note, however, that not all patients with type 1 diabetes develop DN. The cumulative incidence of MA was approximately 30% to 40% at 20 years in a cohort of subjects characterized from the onset of type 1 diabetes,
6 but there appears to be an upper limit of nearly 55%, after 40 years of type 1 diabetes.
29The prevalence of MA in type 2 diabetes ranges in large trials and a global cohort from 25% to 45% after approximately 10 years of diabetes, but may be present at the time of diagnosis of diabetes.
8,
37,
54,
73 The presence of MA, or even overt proteinuria, at the time of diagnosis of diabetes in patients with type 2 DM may reflect the delay in diagnosis of DM, in type 2 as compared to type 1 diabetes. The prevalence of MA varies by age, with older adults more likely to have MA at the time of diagnosis of diabetes,
74 and race; it is highest in Asians and Hispanics and lowest in Caucasians.
54,
75 It was estimated that 2.0% of patients will transition to persistent MA from normoalbuminuria per year (based on data from the United Kingdom Prospective Diabetes Study [UKPDS]).
8 MA is associated with increased CV mortality compared to patients with type 2 diabetes and no MA,
8 with a relative risk for all-cause mortality (which is driven predominantly by CV mortality) of 1.9.
76