Chapter 52 Chronic Pancreatitis
Stones and Strictures
Chronic pancreatitis (CP) is a rare disease in Western countries (incidence of 2 to 10 per 100,000 per year). It ultimately leads to irreversible damage of the pancreas with exocrine and endocrine insufficiency. In the majority of cases pain is the major clinical symptom and is present early in the course of the disease.1,2 With the exception of the tropical and hereditary chronic pancreatitis, the mechanism of CP has not yet been clearly elucidated. Chronic alcoholism is a precipitating factor and dramatically increases the probability of development of CP, but the disease can also develop in nonalcoholic subjects without any obvious genetic background and is then defined as “idiopathic” CP.
The pathophysiology of CP is still debated. Adherents of the “stone theory” believe that the initiating event is protein plug formation due to a congenital lack of lithostatin.3 Proponents of the “necrosis fibrosis” theory, in turn, ascribe fibrosis and ductal stricture as the consequence of focal inflammation and necrosis.4
Pain associated with CP is multifactorial and includes increased interstitial and intraductal pressures, closed compartment syndrome, neural infiltration, ongoing acute pancreatitis, pseudocysts, and biliary obstruction. Elevated intraductal pressure due to the presence of stones and/or strictures is one of the major phenomena leading to pain in CP.5–8 Due to the lack of compliance of the pancreatic gland already present in the early stages of CP, elevated intraductal pressure is quickly associated with increased parenchymal pressure that impairs blood flow, leading to hypoxia, release of oxygen-derived free radicals, and further stimulation of inflammation with subsequent fibrosis.8 Surgical decompression of the main pancreatic duct relieves pain in many patients and is associated with a decrease in intraductal and interstitial pressures.9
Another characteristic of pain in CP is its heterogeneous pattern, from relapsing episodic to persistent pain of varying intensity that cannot be predicted by pancreatic morphology. The initial episodes of acute recurrent abdominal pain or acute pancreatitis often increase and may evolve into a continuous pain syndrome requiring narcotics. During the natural history of CP, pain may disappear after several years, often associated with the development of endocrine and/or exocrine insufficiency.10 This heterogeneous pain pattern is one of the difficulties faced when interpreting results of clinical studies reporting the effectiveness of surgical and endoscopic drainage for pain relief in CP.
Video for this chapter can be found online at www.expertconsult.com.
Endoscopic Treatment: Ductal Decompression by Managing Stones and Strictures
The goal of endotherapy in severe CP is to decompress the main pancreatic duct (MPD) by removing stones and bypassing strictures. Another goal when undertaking MPD drainage is to reduce the incidence or delay the development of steatorrhea by increasing the flow of pancreatic juice into the duodenum. However, whether this can be achieved with endoscopic therapy remains controversial.11,12
Preprocedural Planning (see also Chapter 9)
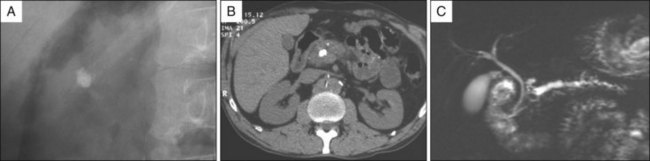
In addition to standard laboratory testing and obtaining plain abdominal radiographs of the pancreatic area or abdominal computed tomography (CT) scan for detection of pancreatic calcifications, magnetic resonance imaging (MRI) is currently the best modality for the selection of patients who may benefit from endoscopic treatment and for planning endoscopic therapy (Fig. 52.1).
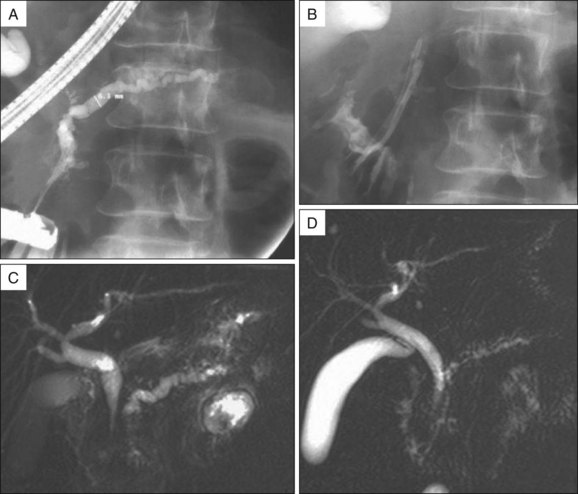
MRI performed with secretin stimulation (S-MRCP) allows information about pancreatic ductal anatomy, presence of peripancreatic fluid collections, and possible obstruction of the bile duct to be determined. Moreover, S-MRCP can be used to quantify pancreatic exocrine function and to evaluate the short- and long-term effects of pancreatic ductal drainage procedures (Fig. 52.2).13,14
MPD Cannulation and Endoscopic Pancreatic Sphincterotomy
Biliary sphincterotomy performed before EPS is performed in the setting of cholangitis or obstructive jaundice or when it is technically necessary to facilitate access to the PD. If a biliary sphincterotomy is performed, the PD orifice is located between the 3 o’clock and 6 o’clock positions on the right margin of the sphincterotomy. After pancreatic opacification, a hydrophilic guidewire (Terumo Inc., Japan; Glidewire, Boston Scientific, Natick, Mass.) can be maneuvered through the stricture or alongside the stones, using a torque device under radiologic guidance (Fig. 52.3). Pancreatic sphincterotomy is then performed over the guidewire after deep cannulation with a standard or tapered pull-type sphincterotome. We prefer to use pure cutting current, extending the incision to the duodenal wall. The same technique can be used for minor papilla sphincterotomy (see Chapters 19 and 20). Alternatively, a PD stent can be inserted into the PD and sphincterotomy performed with a needle knife over the stent.
Extracorporeal Shock Wave Lithotripsy
Technically, it is important to use a lithotriptor with a bidimensional x-ray focusing system and a high-power generator. Ultrasonic localization of pancreatic stones lacks precision. When performed under general anesthesia or deep sedation, 3000 to 6000 shock waves can be applied at an intensity of 0.33 to 0.54 mJ/mm2, which provides complete stone fragmentation after a median of one session, though in our experience up to five sessions may be needed.15,16 With the patient in the prone position the shock wave generator is placed to the patient’s right side when stones are located in the head of the pancreas and to the patient’s left side when stones are located in the body or tail of the pancreas. ESWL is much more effective for fragmentation of calcium carbonate pancreatic stones than for biliary stones. Stones fragmented into millimeter size can usually be easily removed during endoscopic retrograde cholangiopancreatography (ERCP) (Fig. 52.4). When the lithotriptor is located within or close to the endoscopy unit, ESWL and therapeutic ERCP may be performed consecutively during one general anesthetic session.
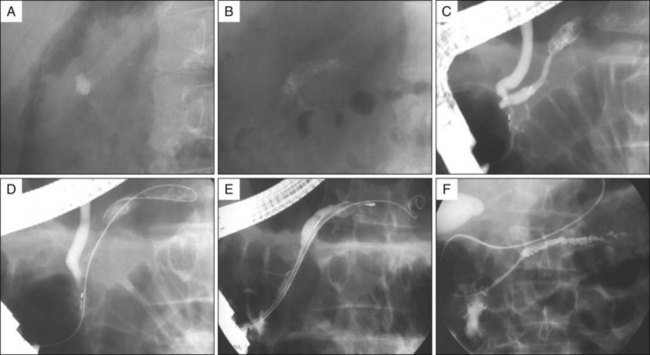
Fig. 52.4 The same patient as in Fig. 52.1. Successful fragmentation (A versus B) after ESWL is illustrated by a decrease in radiologic density, an increase of the stone surface area, and heterogeneity of the stones (powderlike material). After fragmentation a pancreatic sphincterotomy is performed (C), a guidewire is inserted in the pancreatic duct (D), and a small Dormia basket (E) is maneuvered alongside the guidewire to remove the stone fragments. At the end of the procedure a nasopancreatic catheter is left in place (F).
Intraductal Lithotripsy
Some endoscopists have reported successful pancreatic stone fragmentation using intraductal mechanical lithotripsy.17 However, the success of mechanical lithotripsy depends on the ability to capture the stone within the basket, which is most often impossible with impacted, calcified stones. A pulsed dye-laser lithotriptor has also been used to fragment pancreatic stones under direct endoscopic visualization with a pancreatoscope (see Chapter 25) or using fluoroscopic guidance alone.18,19 Laser lithotripsy has proven to be effective in a minority of patients. Intraductal fragmentation remains anecdotal and is not as effective as ESWL. ESWL is the gold standard for pancreatic stone fragmentation because of its efficacy, simplicity, and relative lack of adverse events.
Stone Extraction and Dilation
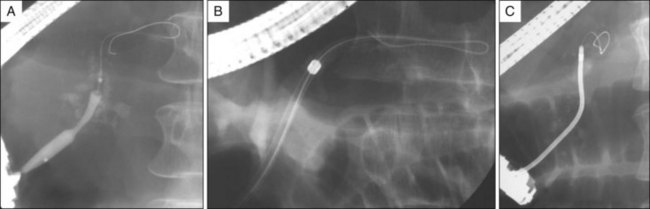
After ESWL, minute stone fragments can be visualized within the PD. If located upstream to a stricture, the stricture is dilated using a 4- to 6-mm balloon (Maxforce, Boston Scientific) to facilitate stone removal. We prefer to use a small Dormia basket to remove stone fragments (Fig. 52.4). When stones are visible on fluoroscopy, a useful trick is to introduce a guidewire into the MPD and then follow with the Dormia basket using minimal or no contrast injection, which allows fluoroscopic localization of residual fragments that become isodense when contrast is injected, and then the basket can be manipulated to trap them. Most often the basket is left opened in the duct, turning on its axis while gently perfusing the duct with saline. A slightly inflated balloon catheter may be used in some cases but is of limited use in the pancreas because sharp stone fragments frequently rupture the balloons. Tight strictures are often present and although balloon dilation is used most frequently, bougies (Soehndra dilators, Cook Endoscopy, Winston-Salem, N.C.) may be necessary; in a case of strictures in which catheter passage proves impossible, a Soehendra screw stent retriever (8.5 Fr) can usually be rotated through the stricture to allow subsequent passage of a dilation balloon (Fig. 52.5).
If multiple endoscopic sessions are necessary for fragmentation and removal of stones, a nasopancreatic catheter (NPC) (see Chapter 21) is left in place for drainage between sessions. This may decrease the risk of acute pancreatitis due to fragment impaction.20 NPC placement can also be used to predict the need for pancreatic stenting; if NPC perfusion is well tolerated without producing pain, an underlying significant MPD stricture duct is unlikely and stent placement may be avoided. In contrast, if NPC perfusion is painful, the catheter should be placed for gravity drainage and further stone extraction or stent placement should be performed.
Stent Placement
When an obstructed MPD stricture is present, adequate outflow from the pancreas to the duodenum must be achieved by PD stent placement. In contrast to stent placement to prevent post-ERCP pancreatitis in high-risk patients (see Chapter 7), multiple and larger diameter (7 to 10 Fr) stents are used. Stent lengths are selected based on pancreatic duct length and stricture location. Our approach is to replace stents every 6 months or “on demand” when symptoms recur; stents remain in place for up to 2 years. Stent placement has evolved from single to multiple side-by-side stents. Two 8.5 Fr stents usually can be placed after a dilation to 6 mm. The number of stents can be further increased over successive exchanges if the upstream ductal diameter of the pancreas allows it (Fig. 52.2). This approach has been used to reduce duration of stenting and prolong symptom relief.21 Multiple stent placement is facilitated by placement of two guidewires across the stricture followed by successive stent placement. This technique avoids the need to recannulate the pancreatic duct and negotiate a guidewire across a tight stricture with a stent already in place.
The use of the Fusion system (Cook Endoscopy) allows intraductal exchange and placement of multiple side-by-side stents without losing access and using only one guidewire (see Chapter 21). This system has become our preferred technique for placement of multiple 8.5 Fr plastic pancreatic stents.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree