12 Botulinum Toxin Injection Therapy
Mechanism of Action
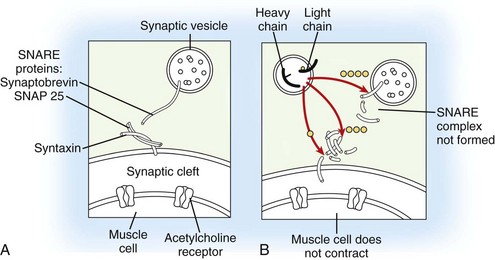
Depending on the serotype of the organism, Clostridium botulinum, seven distinct BoTNs can be produced by the bacterium (types A, B, C1, D, E, F, and G), all with similar mechanisms of action. At the present time, only BoTN A (Botox [Allergan, Irvine, CA] or Dysport [Medicis, Scottsdale, AZ]) and BoTN B (Myobloc or Neurobloc [Solstice Neuroscience, Louisville, KY]) are commercially available for clinical use. BoTN acts by cleaving a specific site (specific to each BoTN serotype) of a protein complex (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor [SNARE] complex) responsible for exocytosis of neurotransmitter vesicles from the neuron. In the case of BoTN A, the most well-studied toxin subtype, the specific substrate is the synaptosomal associated protein of 25 kD (SNAP-25), a component of SNARE complex, which results in the inhibition of synaptic release of acetylcholine from the peripheral motor neuron end plate at the neuromuscular junction and ensuing muscle paralysis (Figure 12-1). More recent experience also suggests toxin effect on neurotransmitters related to sensory (afferent) function in the lower urinary tract.