8 Single-Incision Synthetic Midurethral Slings
Indications and Patient Selection
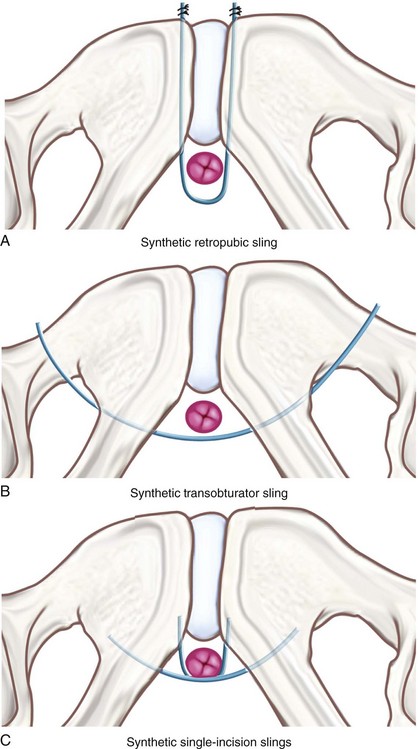
Indications for the single-incision slings are similar to the indications for the more traditional MUS. Because these slings are less invasive than a retropubic or transobturator MUS, they may be desirous for some special patient populations. Because they avoid the retropubic space, the minisling may be considered specifically in patients who have undergone previous retropubic and abdominal procedures and may be at higher risk for significant pelvic adhesions. Because it does not entail complete passage of trocars to the skin level, it may be considered in patients with significant soft tissue mass or obesity in the areas of tradition MUS trocar site exit (i.e., truncal or intertrigonal obesity) that may surpass the length of the trocar. Because single-incision minisling procedures can be done under local anesthesia, they can also be considered in patients with significant comorbidities in whom general anesthesia is contraindicated. At the present time, the authors rarely use single-incision slings in patients with primary SUI because long-term data showing efficacy comparable to retropubic or transobturator MUS are lacking (see Outcomes section).
Description of Various Types of Single-Incision Slings
The MiniArc Single-Incision Sling (Figure 8-1) is a polypropylene mesh (8.5 cm × 1.1 cm) with permanent self-fixating tips that is deployed with a supplied metal 2.3-mm needle/trocar. The mesh is connected to the tip of the needle before insertion; the mesh and needle are inserted; and the needle is removed, leaving the mesh behind. Self-fixating tips are constructed of polypropylene and have two anchoring barbs that help resist up to 5.5 lb of pull-out force to remove the mesh. A redocking maneuver can be set up before insertion to allow retrieval and reinsertion of the mesh if necessary.
The Solyx SIS system (Boston Scientific Corp., Natick, MA; Figure 8-2) includes a polypropylene mesh tape (9 cm in length) with permanent barbed self-fixating tips and a metal and plastic delivery device or trocar. This system is designed similarly to the MiniArc Single-Incision Sling system, in that each tip of the sling is sequentially attached to the end of the delivery device for mesh placement, which is removed after insertion. The edges of the center 4 cm of the mesh (advertised as the suburethral portion) are bonded together to potentially reduce irritation and the possibility of mesh erosion or extrusion.
The AJUST Adjustable Single-Incision Sling (Bard Medical, Covington, GA; Figure 8-3) is a new adjustable minisling that allows the surgeon to tighten or loosen the sling after it has been anchored into the obturator membrane.