Chapter 22 Biliary Metal Stent Insertion
There are two main categories of biliary stents: fixed diameter plastic stents (PS) and self-expandable metal stents (SEMS). SEMS are used primarily for the palliation of malignant biliary obstruction. SEMS can further be classified as uncovered, partially covered, or fully covered. PS, introduced in 1980, preceded their SEMS counterparts and are discussed in detail in Chapter 21. While PS are a safe and effective means to overcome biliary stenoses, they eventually occlude.1 PS occlusion is attributed to biofilm formation and occurs in 30% and 50% of patients within 3 and 6 months, respectively.2 Bile flow rate is a function of stent lumen diameter, which is in turn limited by the accessory channel size of the duodenoscope. Because the diameter of the accessory channel of a therapeutic duodenoscope is 4.2 mm, PS are available with internal diameters of up to 12 Fr (4 mm). SEMS were developed to overcome this limitation, as they deliver a larger-diameter stent (10 mm) via a small-diameter, 7.5 Fr delivery system. Because malignant biliary obstruction is typically associated with a survival of less than 1 year, SEMS are intended to yield “lifelong” palliation of obstructive symptoms, thereby obviating the need for interval endoscopic retrograde cholangiopancreatography (ERCP) for stent exchanges, as would be necessary for PS.3,4
This chapter reviews biliary SEMS, including indications for placement, available types, techniques for placement, avoidance and management of adverse events, and relative cost.5
Video for this chapter can be found online at www.expertconsult.com.
Indications
SEMS are indicated for palliation of malignant biliary obstruction. The most common cause of malignant biliary obstruction is pancreatic adenocarcinoma arising in the head or genu region of the pancreas. Other causes of malignant biliary stenoses are cholangiocarcinoma, ampullary carcinoma, gallbladder cancer, and extrinsic compression associated with lymphadenopathy due to lymphoma and metastatic carcinoma. Patients with malignant biliary obstruction typically present with jaundice. Left untreated, biliary obstruction may cause pruritus, pain, cholangitis, hepatic synthetic dysfunction, and malabsorption. Without therapy, the mean survival for patients presenting with malignant biliary obstruction is <200 days. Because most patients have advanced disease at the time of presentation, operative resection with curative intent is only possible in 10% to 15% of cases.6,7 Therefore palliation of symptoms is a major component of management in the many patients with malignant biliary obstruction (Box 22.1).
Options for palliation of malignant biliary obstruction include operative bypass, percutaneous drainage, and endoscopic stent placement. Multiple studies, including randomized clinical trials, have compared these three biliary drainage outcomes. In a recent meta-analysis of 2436 patients (24 studies), operative bypass, PS, and SEMS were compared.8 While PS were associated with fewer adverse events and shorter hospital stay than operative bypass, there were higher rates of recurrent biliary obstruction. Similar technical and therapeutic success rates, mortality, and adverse events were seen following attempted SEMS placement but with lower rates of biliary obstruction at 4 months than PS (odds ratio [OR] 0.44, 95% confidence interval [CI] 0.3–0.63). While SEMS remain patent longer than PS, their patency is not indefinite.
SEMS versus Plastic Stents
Both PS and SEMS can be used for palliation of malignant biliary obstruction. PS are safe and effective, are markedly less expensive than SEMS, and can be removed and replaced if occlusion occurs. Again, the main drawback of PS is earlier occlusion. Symptoms of stent occlusion include recurrence of jaundice and/or ascending cholangitis. Two strategies have been employed to prevent and manage PS occlusion: (1) prophylactic stent exchange and (2) expectant management. The former involves elective stent removal and exchange at 3-month intervals in order to reduce the risk of cholangitis and need for emergency exchange, while the latter relies on watchful waiting and rapid response should stent occlusion occur. Further discussion on PS can be found in Chapter 21. SEMS were designed to extend the duration of patency owing to their larger internal diameter, thereby reducing the need for and frequency of reintervention. As such, their increased cost may be offset by a reduction in episodes of cholangitis and the need for elective and emergency interventions, including hospitalization.
The multicenter U.S. Wallstent study group performed a trial that randomized patients to uncovered SEMS placement (Wallstent) and 10 Fr PS placement for palliation of malignant distal bile duct obstruction.9 Early stent occlusion due to sludge accumulation occurred in about 3% in the PS group as compared to 0% in the SEMS group. During long-term follow-up the probability of stent occlusion was almost threefold greater for PS than for SEMS. Tumor ingrowth or overgrowth contributed to about 14% of SEMS occlusions and did not occur in the PS group. However, the overall adverse event rate was significantly lower in the SEMS group than in the PS group (20% versus 31%, p <0.05). In the PS group there was a higher number of procedures performed, which resulted in a higher cost. Another prospective randomized trial was performed to compare patency and determine the cost-effectiveness of SEMS versus PS.4 Median patency was significantly longer for the SEMS group at 273 days compared with a median of 126 days for the PS group. Two subsequent prospective randomized trials10,11 also showed longer duration of patency with uncovered SEMS compared to PS. Along with the extended patency rate there were significantly fewer accumulated hospital days in the SEMS group.
Concerns about the use of SEMS prior to confirming a tissue diagnosis of cancer and unresectability have been more recently contested. It is important to note that short-length uncovered SEMS have been placed preoperatively to relieve biliary obstruction in patients with resectable pancreatic adenocarcinoma. The benefit of preoperative SEMS placement is debated.12 Preoperative biliary drainage may alleviate jaundice and cholestasis-associated adverse events and allow time for delivery of neoadjuvant chemoradiation. However, it may also increase cost and the risk for procedure-related adverse events. While there had been concern about SEMS complicating operative resection, this has not been borne out in clinical practice. Several series have been reported to date detailing the utility of preoperative drainage with SEMS in resectable pancreatic adenocarcinoma. Thus far, these series have consistently reported that preoperative SEMS placement does not impose surgical technical difficulties or influence postoperative course or long-term outcome. Studies have also indicated that for preoperative indications SEMS require fewer endoscopic interventions than PS.12–15 A Monte Carlo decision analysis that compared several preoperative strategies in patients with resectable distal pancreaticobiliary cancer concluded that placement of short-length biliary SEMS provided equal or superior efficacy and reduced overall costs compared to PS placement.15 While these published series are neither prospective in nature nor randomized, there is sufficient evidence to support the selective placement of SEMS prior to anticipated operative resection when considered on an individualized basis.
SEMS for Benign Biliary Disease
While SEMS placement has traditionally been used for palliation of malignant bile duct obstruction, a growing body of literature is emerging pertaining to the use of fully covered (and therefore anticipated as removable) SEMS for management in selected patients with benign biliary conditions (strictures, leaks, fistula, postsphincterotomy bleeding).16–18 Benign strictures can be due to postoperative injury, anastomotic, or related to chronic pancreatitis, primary sclerosing cholangitis, and gallstones (see Chapter 40). Covered SEMS have shown promise in the treatment of benign biliary strictures and possibly bile leaks with the advantage of fewer procedures (which offsets the initial high stent cost), but more conclusive data are necessary to define their role in the management of benign biliary diseases. The use of SEMS in liver transplant recipients for the treatment of bile leaks has been reported to be associated with high rates of subsequent de novo stricture formation (see Chapter 41).17,19,20
Types of SEMS
Covered versus Uncovered SEMS
Uncovered SEMS are associated with lower rates of stent migration and can be used anywhere in the bile duct including the hilum.21–23 However, tumor ingrowth and limited removability are limiting factors of uncovered SEMS. Covered SEMS were developed to overcome tumor ingrowth and tissue hyperplasia through the SEMS interstices. Covered SEMS share the same indications as their uncovered counterparts, though they are not advocated for hilar or intrahepatic obstruction because they may block the contralateral intrahepatic system or ipsilateral intrahepatic branches. The anticipated advantage of covered SEMS is the prevention of tissue ingrowth (malignant or hyperplastic) and subsequent stent occlusion while allowing for potential removability (although these stents are approved by the Food and Drug Administration [FDA] for immediate—not delayed—removal). While there are more published data on the use of uncovered stents, there is a growing body of literature advocating the use of partially covered SEMS. Studies of covered SEMS have demonstrated very low rates of stent occlusion due to tumor ingrowth.24,25 However, concerns have been raised about higher rates of stent migration and stent-induced cholecystitis and pancreatitis from cystic duct obstruction and pancreatic duct obstruction, respectively.24,26–28
A meta-analysis evaluating stent patency of covered and uncovered SEMS in unresectable distal bile duct obstruction encompassing five randomized clinical trials of 781 patients concluded that covered SEMS have longer patency rates but higher rates of stent migration and sludge formation than uncovered stents.29 The results are interpreted with caution given the limited number of studies. More recently, several randomized controlled trials of partially covered versus uncovered SEMS have been published.30,31 The major findings of these studies are a significantly increased rate of stent migration with covered stents, reported to be between 3% and 13%. Cholecystitis occurred in 1% to 7% and there was no significant difference between the two groups. Pancreatitis rates were low (≤2%) and did not differ between the two groups. Most importantly, there was no significant difference in stent patency or patient survival between the partially covered and uncovered stent study arms.
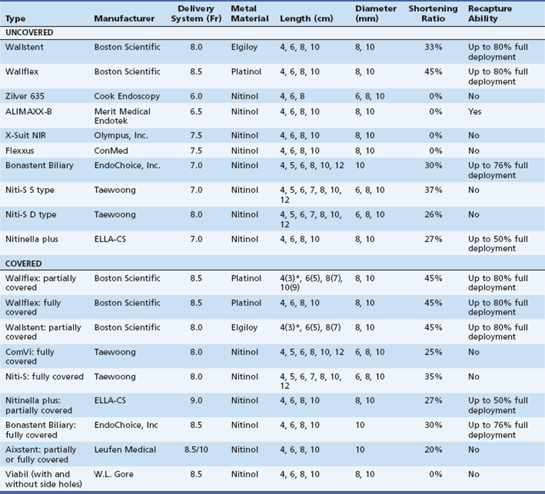
There are a variety of commercially available SEMS used in the palliation of malignant biliary obstruction (Table 22.1). They vary in design, delivery system, configuration, mechanical properties, type of metal, size, and price; all of them can be deployed through a duodenoscope. There are few studies comparing different SEMS. While stents are constantly being introduced into the marketplace, the following are the most commonly available uncovered stents: Wallstent (Boston Scientific, Natick, Mass.), Wallflex stent (Boston Scientific), Zilver stent (Cook Endoscopy, Winston-Salem, N.C.), ALIMAXX-B stent (Merit Medical Endotek, South Jordan, Utah), X-Suit NIR biliary stent (Olympus, Center Valley, Pa.), Flexxus stent (ConMed, Billerica, Mass.), Niti-S S type and Niti-S D type (Taewoong, Seoul, South Korea), Nitinella plus (ELLA-CS, Hradec Králové, Czech Republic), and Bonastent Biliary (EndoChoice, Inc., Alpharetta, Ga.). Covered stents include the covered (partially) Wallstent (Boston Scientific), Viabil stent (W.L. Gore, Flagstaff, Ariz., distributed by ConMed), Wallflex (Boston Scientific), ComVi and Niti-S (Taewoong), Nitinella (ELLA-CS), and Bonastent Biliary (EndoChoice, Inc.). The following sections describe some of the most commonly used SEMS in clinical practice.
Uncovered SEMS
Wallstent
The Wallstent was the first SEMS available for endoscopic use and is considered the industry standard (Fig. 22.1). The majority of data on SEMS outcome applies to the biliary Wallstent (Boston Scientific), a braided stainless steel (Elgiloy) mesh with barbed ends. The uncovered Wallstent is available in 4-, 6-, 8-, and 10-cm lengths. The available fully expanded diameters are 8 and 10 mm. The delivery device has an outside diameter of 8 Fr and consists of a 0.035-in guidewire-compatible introducer catheter on which the compressed SEMS is constrained by a hydrophilic-coated outer sheath. The Wallstent also is available in a partially covered (silicone) form; the outermost ends are uncovered for 0.5 mm with the same delivery device and diameter as its uncovered counterpart. The delivery device has a tapered tip to facilitate insertion. The SEMS is deployed by withdrawing the outer sheath. The Wallstent is radiopaque and has four radiopaque markers on the delivery device to guide precision deployment. The stent can be recaptured, if need be, and repositioned until 80% of full stent release. Wallstents can be deployed entirely within the bile duct or in a transpapillary position. There is 33% foreshortening of the Wallstent during insertion and continuing until fully deployed (which may be several days after insertion). Transpapillary positioned uncovered Wallstents may be reliably removed within 12 to 24 hours after insertion. After 24 hours, the uncovered stent becomes embedded in the bile duct wall and is difficult, if not impossible, to remove.
Wallflex
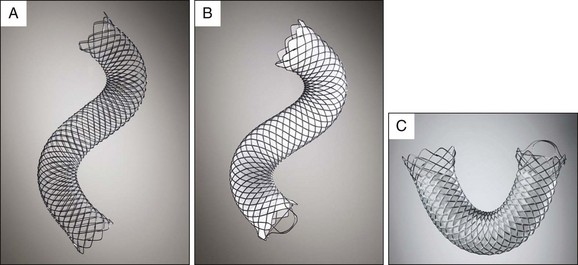
The uncovered Wallflex is constructed with a closed loop platinum core nitinol wire (Platinol) mesh with looped and flared ends designed to be less traumatic to the epithelium than the open ends of the Wallstent. The end of the stent has a retrieval loop to allow for its removal. The platinum core enhances fluoroscopic visualization and flexibility to conform to anatomic variations and angulations without sacrificing intraluminal diameter or radial force. The uncovered Wallflex has an 8.5 Fr delivery system and is available in 4-, 6-, 8-, and 10-cm lengths and in 8- or 10-mm diameters. The Wallflex is also available in partially covered (silicone) and fully covered versions (Fig. 22.2, A).
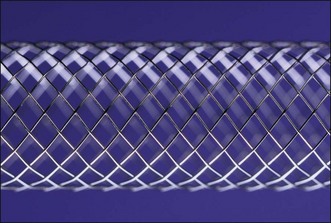
Zilver
The Zilver stent (Fig. 22.3) is a single tube of nitinol that is laser cut compared to a single strand of woven nitinol (e.g., Wallflex, Wallstent). This results in an open cell–design stent rather than the tight weaving or interlocking of closed cell–design stents. The gold radiopaque markers are at the proximal and distal end of the stents. The introducer diameter is 6 Fr, which is the smallest on the market. This allows passage of side-by-side predeployed stents though the working channel of a therapeutic channel duodenoscope for hilar placement. The stents are subsequently deployed.32 The release mechanism is similar to that of the Wallstent. The Zilver stent is nonshortening, which facilitates accurate deployment.
Niti Stents
A group of stents made by Taewoong include the Niti series. These stents are composed of nitinol wire and are available in multiple formats. The Niti-S D type stent has a “hook and cross” structure that results in a D configuration of the wires at deployment. Its design allows for conformability and flexibility of the stent, minimal stent shortening, and maximal radial force, which makes it suitable for hilar obstructions. The Niti-D stent has been compared to the Wallstent with similar stent patency rates and outcomes for distal malignant biliary obstruction.33
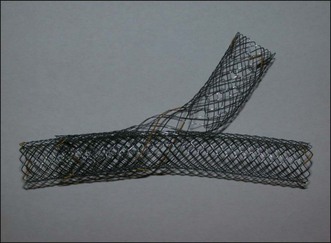
Other Niti type stents include the Niti-S biliary uncovered, Niti-S biliary Y, and Niti-S large cell D type biliary stents. The Niti-S biliary uncovered stent features atraumatic ends and limits tissue hyperplasia. The Niti-S biliary Y stent has a central wide open mesh region through which a second stent may be placed into the contralateral hepatic duct for palliation of malignant hilar obstruction (Fig. 22.4).34 Finally, the Niti-S large cell D type biliary stent uses a thicker nitinol wire (0.178 mm) and larger cell size to achieve low axial force while allowing a second stent to be passed through the mesh. This stent can be used in combination with another stent for hilar obstruction.
Flexxus
The Flexxus stent (formerly Memotherm and then Luminexx, by ConMed) is a highly flexible nitinol stent with flared ends (Fig. 22.5) and is also laser cut. The interstices are large enough to permit cannulation with a wire and passage of a second stent to create a Y configuration for palliation of hilar strictures.35 There are four metal (Tantalum) markers on each end to improve fluoroscopic imaging. The predeployment delivery diameter is 7.5 Fr and the postdeployment diameters are 8 and 10 mm with lengths of 4, 6, 8, and 10 cm. The release mechanism is unique and employs a pistol-grip handle that withdraws the constraining sheath and allows stepwise, controlled release. During deployment there is no foreshortening and the stent cannot be reconstrained. A study that compared Wallstents and Flexxus stents demonstrated comparable efficacy, duration of stent patency, occlusion rates, and adverse events.36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree