Endometrial Ablation
Michael S. Baggish
Rafael F. Valle
Intractable uterine bleeding unresponsive to conservative hormonal therapy or dilatation and curettage has in the past been managed by hysterectomy (Table 25.1). The VALUE National Hysterectomy Study from the United Kingdom obtained data from 276 National Health Service hospitals comprising 37,298 cases. The main indication for hysterectomy in this series was dysfunctional uterine bleeding and numbered 16,100 cases. As noted in our second edition, beginning in 1981, a reasonable alternative to hysterectomy appeared in the form of transcervical hysteroscopic endometrial ablation (Table 25.2). In a recent study comparing 64 women who underwent endometrial ablation and 46 women who underwent hysterectomy, the operating time was 38 minutes versus 107, hospital stay 0.7 versus 2.7 days, and complications 6.3% versus 21.7%. Total costs for endometrial ablation averaged $5,959 versus $11,777 for hysterectomy.
Techniques
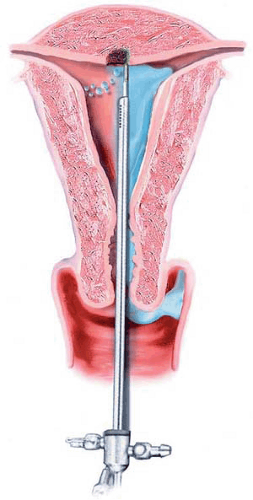
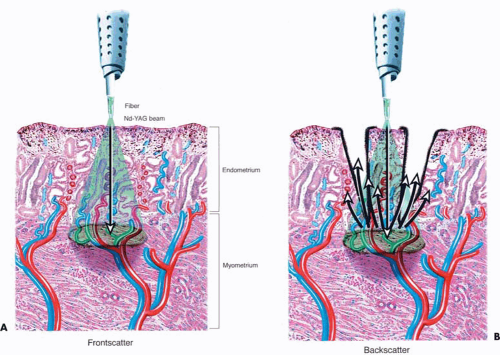
During the 1980s and the early 1990s, most ablations were performed by means of the Nd-YAG laser (neodymium–yttrium-aluminum-garnet (Fig. 25.1). The 600- to 1,000-micron quartz fiber delivery system was inserted through the operating channel of the hysteroscope and touched to the surface of the endometrium (Fig. 25.2). As laser power was applied, ranging from 40 to 100 watts, the fiber was dragged toward the hysteroscope thereby ablating (vaporizing) the endometrium in a furrowlike action (Fig. 25.3A, B).
During the past decade, electrosurgery has largely replaced the laser as the technique for accomplishing endometrial ablation. The resectoscopic electrode has replaced the laser fiber as the most common delivery system. Two general variations of the operation have evolved: ball electrode (roller-ball) endometrial ablation and loop-electrode (resecting loop) endometrial resection. Interestingly, most ablations performed in the United States are via roller-ball whereas the resection technique has gained the most popularity in Europe. The major differences between the two electrosurgical techniques are as follows: (a) The ball electrode coagulates tissue, and the depth of tissue necrosis is less than the depth of tissue removed with the cutting-loop electrode; (b) postoperative hemorrhage and perforation are more prevalent with endometrial resection; (c) one or more specimens are obtained as a result of endometrial resection. However, the outcomes are similar. In a 2001 study published in the Journal of Reproductive Medicine, endometrial resection had an 8.6% perforation rate including one case with collateral bladder and ureteral trauma. The end result of these ablation procedures was to produce significant scarification of the endometrium, rendering the cavity to be shrunken and deformed. In some cases, severe Asherman disease was produced.
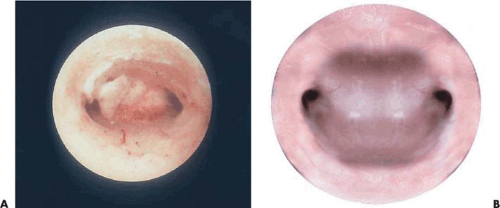
The goal of ablation/resection surgery is to create amenorrhea by wiping out all the functioning endometrium. Immediately following ablation/resection, amenorrhea may approach an incidence of 80% to 90% (Fig. 25.4A–C). In practical terms, long-term amenorrhea will be achieved in approximately 50% of the patients under the best-care scenario, whereas some degree of amenorrhea and hypomenorrhea will be seen in 80% to 90% of those treated by an experienced, skilled hysteroscopic surgeon. Combining ball electrode ablation with endometrial resection offers no advantages relative to outcomes and may actually increase the risk of untoward outcomes.
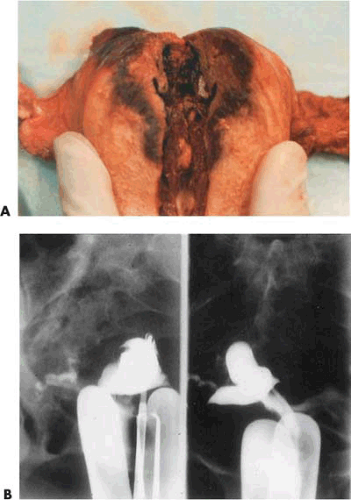
Goldrath (1981) originally described an Ashermanlike cavity following laser ablation. Baggish in early hysteroscopic follow-up examination of 30 women (3 months) found the cavity to be intact but having a shrunken white appearance.
DeCherney and Polan in 1983 described a method of resecting the endometrial surface using the wire loop and the trigger mechanism of the cystoscopic resectoscope. This procedure was performed on 11 patients in less than 30 minutes each, with two-thirds of a high-risk group of women attaining long-term amenorrhea. In a later report, the New Haven group reported on 21 patients who underwent endometrial cautery excision in which Hyskon was used as the distending medium. Eighteen patients followed for >6 months had no further vaginal bleeding. Interestingly, five patients had significant immediate bleeding, necessitating insertion of a Foley catheter into the uterus for up to 24 hours postoperatively. Three patients in the earlier series required an intrauterine Foley catheter for approximately 6 hours postoperatively to stop bleeding.
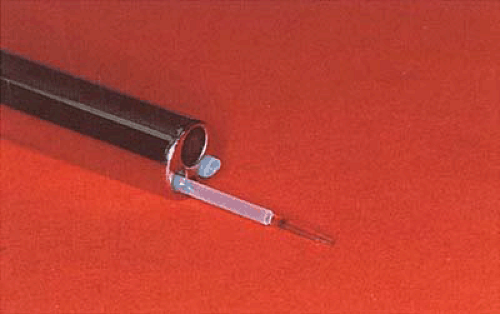 FIGURE 25.1 Isolated channel hysteroscope with a sculpted laser fiber measuring approximately 1,000 μm. The second operating channel contains an aspiration cannula. |
Baggish and Baltoyannis reported a series of high-risk women in whom hysterectomy would have been a life-threatening operation and in whom Nd-YAG laser surgery was used to stop heavy, uncontrolled bleeding that had led to anemia. Half of these patients were diagnosed as having a major clotting defect.
TABLE 25.1 Number and Rates of Hysterectomies by Year, in United States 1975–1989 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
Several large published series have appeared in the literature. Garry et al. (1995) reported 600 endometrial laser ablations in which no major operative morbidity was encountered. The overall success rate was 83.4%. Magos et al. (1991), also from the United Kingdom, reported 250 cases of endometrial resection with a 92% improvement of abnormal
uterine bleeding. Baggish and Sze performed a total of 591 ablations between 1993 and 1995 and reported data on 568 cases. From 1983 to 1991, 401 operations were done with the Nd-YAG laser. From 1991 to 1999, a 3-mm monopolar ball electrode connected to an electrosurgical unit has been the instrument of choice for this operation (190 procedures). Total amenorrhea was observed in 58% of the women treated and light menstrual flow in 31%. The overall success rate was 89%.
uterine bleeding. Baggish and Sze performed a total of 591 ablations between 1993 and 1995 and reported data on 568 cases. From 1983 to 1991, 401 operations were done with the Nd-YAG laser. From 1991 to 1999, a 3-mm monopolar ball electrode connected to an electrosurgical unit has been the instrument of choice for this operation (190 procedures). Total amenorrhea was observed in 58% of the women treated and light menstrual flow in 31%. The overall success rate was 89%.
TABLE 25.2 Hysteroscopic Laser Ablation of the Endometrium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Two large randomized controlled studies compared hysterectomy with hysteroscopic ablation/resection. Dwyer et al. compared prospectively 100 women undergoing endometrial resection and 100 women having abdominal hysterectomy for menorrhagia. Postoperative morbidity, length of hospital stay, and time taken to return to work, normal daily activities, and sexual intercourse were significantly less in the endometrial resection group. Dysmenorrhea and premenstrual symptoms were significantly higher in the endometrial resection group. Pinion et al. randomized patients to abdominal or vaginal hysterectomy (99), endometrial laser ablation (53), and endometrial resection (52). Women treated by ablation or resection had less morbidity and a shorter recovery. After 12 months, 89% of patients in the hysterectomy group and 78% in the hysteroscopy group were very satisfied with the effect of surgery. Some 95% and 90%, respectively, thought that there had been acceptable improvement in symptoms. Equal numbers would recommend the same operation to others.
TABLE 25.3 Endometrial Resection | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the Baggish and Sze series, the principal indication for endometrial ablation was abnormal uterine bleeding. This was broadly defined in an earlier publication. During the first 2 years of this study, the operation was limited to only those women who would not safely tolerate hysterectomy. A total of 57 women had significant associated medical disorders including bleeding diatheses and therapeutic anticoagulation. Fifty-five women had ablation because of morbid obesity plus abnormal bleeding. Twenty-nine women had a concurrent submucous myoma. The myoma was destroyed at the time of ablation. The remaining cases were done for abnormal bleeding without other comorbidities.
Patient Selection
Hysteroscopic ablation of the endometrium is a relatively noninvasive method that can accomplish the treatment without removing the uterus, requires less hospitalization, and involves less disability to the patient; however, it should be reserved for patients who cannot be treated by other simpler methods such as medical treatment. Most such patients have dysfunctional uterine bleeding and are therefore amenable to hormonal management.
Indications/Contraindications
Uterine Enlargement
Uterine size is a predictor of success. Uteri >10 weeks’ gestational size have a better prognosis relative to amenorrhea, hypomenorrhea, and oligomenorrhea and lower risk for subsequent hysterectomy. For those women with large uteri, i.e., >10 weeks’ gestational size, ablation can technically be performed but hysterectomy is a better choice for such cases.
Adenomyosis
Dysmenorrhea and adenomyosis are together or singly poor prognostic factors for long-term success of endometrial ablation or resection. In the case of resection, dysmenorrhea may appear de novo following surgery. Mints et al. reported on 104 patients who underwent endometrial resection for severe menorrhagia with mean follow-up of 29 months. Eleven percent of these women developed postoperative long-term dysmenorrhea. Data published by Raiga et al. from Clermont-Ferrand, France, strongly advocated the exclusion of women with symptoms and signs of adenomyosis and women with enlarged uteri. The work of David Shelley-Jones et al. further supports the exclusion of women with dysmenorrhea and/or endometriosis (Table 25.4).
Hyperplasia and Neoplasia
Endometrial hyperplasia and endometrial cancers are contraindications to ablation/resection. Benign (cystic) hyperplasia is a relative contraindication whereas atypical
hyperplasia and adenocarcinoma are clear contraindications as is endometrial sarcoma. Valle and Baggish (1998) reported eight detailed cases of endometrial carcinoma following endometrial ablation and reviewed the literature. Simple endometrial hyperplasia not responsive to reversal by progestins was found to be a risk factor. Ablation should be avoided in circumstances when hysterectomy is the preferred treatment (Table 25.3).
hyperplasia and adenocarcinoma are clear contraindications as is endometrial sarcoma. Valle and Baggish (1998) reported eight detailed cases of endometrial carcinoma following endometrial ablation and reviewed the literature. Simple endometrial hyperplasia not responsive to reversal by progestins was found to be a risk factor. Ablation should be avoided in circumstances when hysterectomy is the preferred treatment (Table 25.3).
TABLE 25.4 Factors Adversely Affecting the Success of Endometrial Ablation | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Age
Gemer et al. (2003), Garry et al. (1995), and Shelley-Jones et al. (1994) observed that women older than 40 years of age at the time of their endometrial ablation had a significantly greater amenorrhea rate than did younger women. Additionally, the group older than 40 years had a lower risk for subsequent uterine surgery, e.g., repeat endometrial ablation/resection and hysterectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree