Common cause
Uncommon cause
1. Small bowel-related disease
– Internal hernia
– Petersen’s hernia
– Mesocolic hernia
– Mesenteric hernia
– Incisional hernia
– Trocar site hernia
– Adhesive small bowel obstruction
– Intussusception
– Stenosis or leak of the jejunojejunostomy
2. Gastric pouch and gastric remnant-related disease
– Leak
– Ulcer disease (marginal ulcer and ulcer in remnant stomach)
– Stenosis
– Gastrogastric fistula
– Gastroesophageal reflux disease
– Hiatus hernia
3. Biliary disease
– Gallstone (GS)
– Cholecystitis
– Choledocholithiasis
– Cholangitis
– GS pancreatitis
– Sphincter of Oddi dysfunction
4. Functional disorders
– Constipation, diarrhea
– Irritable bowel syndrome
– Dumping syndrome
– Esophageal motility disorders
5. Behavioral and nutritional disorders
– Maladaptive eating behavior: Overeating, rapid eating
– Food intolerance
– Micronutrient deficiencies
– Bacterial overgrowth in the defunctionalized stomach or small intestine
6. Other
– Omental torsion and/or infarction
– Superior mesenteric syndrome
– Median arcuate syndrome
This chapter focuses on the work-up of abdominal pain after LRYGB or LSG while subsequent chapters will discuss complications related to LAGB and the management of specific bariatric procedure-related complications.
8.1.1 Clinical Work-Up of Bariatric Surgical Complications
When a bariatric surgery patient presents with acute abdominal pain, evaluation should follow a stepwise approach. To begin, one must obtain a detailed history and perform a standard physical examination with special attention to the patient’s vital signs. Since a complete evaluation should focus on bariatric surgery-related complications, consultation with a bariatric surgeon should be obtained early in the course of the evaluation. Ideally, abdominal pain work-up should involve the original surgeon as patients are not always aware of the details of their procedure, and variability in surgical technique is ubiquitous. An understanding of the specific bariatric procedure and its potential complications is essential to reveal the diagnosis [5–8] (Fig. 8.1).
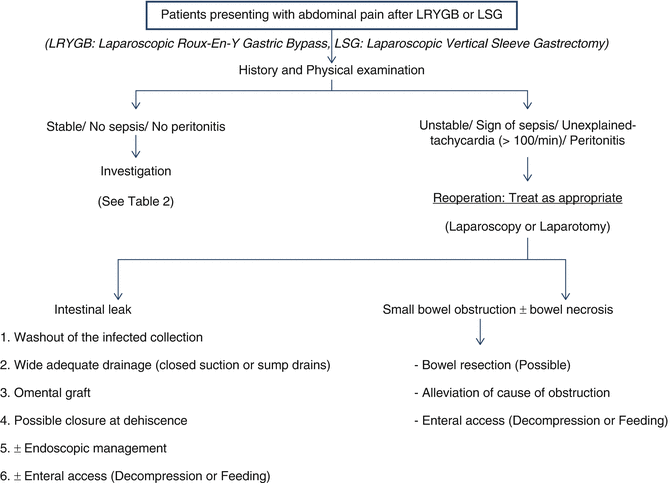

Fig. 8.1
Algorithm of patients presenting with abdominal pain after LRYGB or LSG
Given the broad differential diagnosis in the stable and non-peritoneal patient, diagnostic algorithms must be guided by clinical history and physical exam but should remain flexible. A careful dietary and food history along with serum chemistries, complete blood count, and vitamin levels may reveal behavioral or nutritional causes of pain that are often easily treated. For example, patients may not sense satiety until the gastric pouch has distended to an uncomfortable and often painful state. Small, frequent meals may alleviate these problems, although patients typically learn to recognize early signs of discomfort in the months following surgery and adjust their eating habits accordingly. Additionally, hygroscopic foods such as rice, bread, and pastas should be limited as they are common culprits for uncomfortable gastric distention.
Also common to both gastric bypass and gastric sleeve patients is dehydration. The secondary effects of decreased fluid intake may lead to constipation with lower abdominal colicky pain being the common presenting complaint. Improved hydration and laxative use should alleviate these symptoms.
In any postoperative patient, common causes for abdominal pain may be as simple as a seroma or hematoma formation either in the subcutaneous tissues or the intra-abdominal compartment. Alternatively, one may develop a local wound infection, which has been reported in 3 % of LRYGB versus over 6 % of open RYGB patients [9]. These present with skin-level erythema, fluctuance, induration, pain, and possibly fevers or chills with leukocytosis on laboratory work-up. Local wound care sufficiently treats these; however, if there is a deep or intra-abdominal abscess, image-guided catheter placement versus surgical washout may be warranted.
Additional clues towards obtaining a diagnosis may be obtained through appropriate diagnostic testing (Fig. 8.1 and Table 8.2). Most patients will require abdominal X-rays, upper gastrointestinal (GI) contrast studies, and upper GI endoscopy as useful tests to provide a diagnosis in most cases. If a diagnosis cannot be identified, computerized tomography (CT) imaging of the abdomen and pelvis with intravenous and oral contrast may be indicated. If CT is non-diagnostic, ultrasound or esophageal manometry may be considered depending on the clinical presentation.
Table 8.2
Treatment for stable/no sepsis/no peritonitis patients presenting with abdominal pain after LRYGB or LSG (continue)
1. Complete history and physical examination, focusing on type of operation and presenting symptoms 2. Diagnostic (possible therapeutic) endoscopy 3. Laboratory: Full set of blood work such as CBC, coagulations, liver function, amylase 4. Diagnostic imaging such as acute abdominal series, upper gastrointestinal (GI) contrast study, abdominal ultrasound (US), computer tomography (CT) scan abdomen and pelvis with IV/oral contrast 5. Diagnostic laparoscopy |
The diagnostician must consider the possibility of sepsis caused by anastomotic leak or from necrotic small bowel due to an internal hernia. An intra-abdominal infection from a leaking anastomosis is the most common cause of mortality within the first 12 weeks after surgery [10]. Fever, hypotension, tachycardia, tachypnea, decreased urine output, and hypoxia (with tachycardia being the most sensitive sign [11] should alert the physician to a possible bariatric surgery-related cause of sepsis. In a review by Bellorin et al., an anastomotic leak was likely to be present in patients with sustained tachycardia above 120 beats per minute (bpm) whereas bleeding complications were revealed by cyclical spikes of tachycardia usually less than 120 bpm [12]. The delay in onset between peritonitis and reoperation is the most important determinant of morbidity and mortality.
In general, when evaluating and managing patients who present with abdominal pain, some general guidelines may be observed:
1.
Avoid placing the severely obese patient in a fully supine position during evaluation to minimize possible respiratory embarrassment caused by excess abdominal mass.
2.
3.
Nasogastric or orogastric intubation should be performed only if necessary, and care should be taken to avoid injury due to the altered anatomy of the upper GI tract.
4.
Prolonged use of drugs that may induce gastric mucosal damage (NSAIDs, ASA, and steroids) should be avoided if possible.
5.
The possibility of thiamine deficiency, due to vomiting, acute or chronic malnutrition, or altered eating habits, must be considered. If fluid replacement is indicated, start infusing non-glucose-containing solutions (normal saline or Ringer lactate), and administer thiamine before infusing glucose to avoid an acute onset of Wernicke’s syndrome [15, 16].
The possibility of acute cholecystitis or symptomatic choledocholithiasis should be considered in any patient presenting with right upper quadrant pain after bariatric surgery . A recent meta-analysis revealed that cholecystectomy was subsequently performed in 6.8 % of all LRYGB patients, as compared to 1–5 % of the general population. Of the 6.8 %, 5.3 % were for biliary colic or biliary dyskinesia and 1 % due to cholecystitis [17]. Ultrasound will diagnose gallstones with an accuracy of more than 95 % and nuclear cholescintigraphy will diagnose acute cholecystitis with an accuracy of more than 90 % [18]. More sophisticated endoscopic and laparoscopic-assisted interventions to study the biliary tree or remnant stomach may be necessary in patients suspected of having disease in these organ systems, again keeping in mind the post-surgical anatomic alterations.
If a diagnosis is still not made after taking a full history, lab studies, and imaging, strong consideration should be made for diagnostic laparoscopy. This will allow for diagnosis of some pathologies like internal hernia which may not be evident even after a thorough preoperative work-up. In the very stable patient with a more chronic presentation, conservative therapies including acid suppression medications, smoking cessation, and NSAID avoidance should be considered as adjunctive management. If such conservative therapy fails after 4–8 weeks, diagnostic laparoscopy will likely be required to assess for a potential intra-abdominal source [19]. Unfortunately some may experience persistent pain despite exhaustive work-up and pain management consultation can often provide relief for these patients.
8.2 Procedure-Specific Complications
8.2.1 Small Bowel Obstruction After Bariatric Surgery
The incidence of small bowel obstruction (SBO) following open bariatric surgery has been reported to range from 1 to 5 % [20]. Similar rates have been reported with the laparoscopic approach (0.6–3.9 %) [21]. In a recent review of nearly 10,000 laparoscopic gastric bypasses, Martin et al. reported an overall incidence of 3.6 % [22]. Patients may present with severe intermittent diffuse abdominal pain lasting hours without a relationship to food. Bilious emesis is common, with obstipation being a less common finding, as these are usually proximal obstructions. Unlike open bariatric procedures where adhesive disease is the most common cause of obstruction, SBO after laparoscopic bariatric surgery is caused primarily by non-adhesive disease. An internal hernia is widely recognized as one of the most frequent causes of SBO (>50 %) in bariatric patients. Additionally, abdominal wall hernias may also cause pain in obese patients [22]. Understandably, it may be difficult to identify small incisional or trocar site hernias in an obese patient due to the limitations of physical examination in this population. There are three classic locations where SBO due to internal herniation can occur after LRYGB: Petersen’s space (between the Roux limb’s mesentery and transverse mesocolon in a retrocolic bypass ), at the transverse mesocolon defect (for a retrocolic bypass), and at the jejuno-jejunostomy. Nasogastric decompression may be ineffective on a substantial portion of the gastrointestinal tract (gastric remnant, biliopancreatic limb) and prolonged non-operative management may be futile and dangerous. It is critical to remember that internal hernia often presents with abdominal pain but without bowel obstruction; the pain is caused by bowel ischemia secondary to venous outflow occlusion.
Other causes of post-bypass surgery SBO involve the formation of mesocolic defect strictures around the Roux limb (in retrocolic gastric bypass only), anastomotic strictures, intussusception, and volvulus of the gastric sleeve or the bowel distal to the Roux limb at the J-J anastomosis in LRYGB.
The patient’s diagnosis is based on clinical presentation, radiologic imaging (upper gastrointestinal series or CT), and upper endoscopy. CT scan is an extremely effective diagnostic tool in the bypass population as it can reveal dilatation due to obstruction in the Roux limb, the gastric remnant, or the biliopancreatic limb; in a patient with internal hernia, it may even show a mesenteric “swirl” sign. CT scan has a sensitivity ranging from 78 to 100 % and specificity of 80–90 % [23, 24]. The cardinal signs of obstruction are proximally dilated bowel (usually including the esophagus and gastric pouch), distally collapsed bowel (distal small bowel and colon), and a transition point somewhere in between. Internal herniation is typically represented by the herniated bowel seen as fluid-filled dilated loops of small bowel situated at the left upper quadrant associated with a proximally dilated esophagus/gastric pouch/gastrojejunostomy and distally decompressed small bowel [25]. The high frequency of negative imaging may be due to the fact that CT scans may not be obtained during an episode of incarceration or that incarceration of a short segment of the biliopancreatic limb may not cause recognizable small bowel dilation. For these reasons, severe abdominal pain in a patient with prior gastric bypass is strongly suggestive of internal hernia and mandates surgical exploration unless a clear alternative diagnosis is established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








