Fig. 12.1
Stomal stenosis on endoscopy. With permission from Go MR, Muscarella P, 2nd, Needleman BJ, Cook CH, Melvin WS. Endoscopic management of stomal stenosis after Roux-en-Y gastric bypass. Surg Endosc. 2004;18(1):56–9 [18]. © Springer
Post-bypass GJ strictures can be endoscopically graded into four groups [19]:
Grade I: Mild stenosis—10.5 mm endoscope can be passed
Grade II: Moderate stenosis—8.5 mm pediatric endoscope can be passed
Grade III: Severe stenosis—only a guide wire can be passed
Grade IV: Complete/near-complete obstruction—non-traversable
A pooled analysis by Markar et al. found that 21 mm stapled GJ anastomoses were associated with an increased symptomatic stricture rate compared to 25 mm anastomoses [20]. However, no significant weight loss difference was found between the two groups [20]. Their study concluded that the 25 mm circular stapler would reduce the risk of GJ symptomatic stricture while providing adequate weight loss [20]. A hand-sewn technique using the linear stapler with transverse enterotomy closure has also been associated with a lower stricture rate compared to a vertical longitudinal closure or the 21 mm circular stapler [14, 16, 21]. In general, ischemic strictures are more frequently reported with circular stapled rather than hand-sewn anastomoses [16]. The use of a circular stapler has also been found to be associated with stricture recurrence [17]. However, proponents of circular stapled anastomoses argue that stoma sizes remain relatively constant compared with linear stapled or hand-sewn stomas, which can dilate over time [16].
The treatment of GJ strictures has varied from non-operative endoscopic dilation to open or laparoscopic surgical revision of the gastrojejunostomy combined with medical management to prevent future recurrence [13, 19]. The first-line treatment for a GJ stricture is usually endoscopic balloon dilation, which has been shown to be a very effective strategy. Early endoscopic intervention is important in RYGB patients with GJ strictures in order to alleviate symptoms and avoid complications such as dehydration and metabolic derangement. A shorter interval from surgery to initial dilation has been associated with a higher likelihood of success and, accordingly, a lower likelihood of requiring revisional surgery. Early strictures are very responsive to endoscopic dilation because they usually result from simple mucosal overgrowth [16]. In fact, most patients require only a single dilation when presenting early after surgery or less than 90 days.
Serial balloon dilation every 2–3 weeks to a maximum of 15 mm has been shown to resolve symptoms with an overall success rate of over 80 % [14]. Although the optimal maximal dilation size still remains to be determined, over-dilation should be avoided to prevent complications such as perforation and preserve weight loss [15]. Ryskina et al. found that balloon dilation up to 15 mm was not associated with reduced weight loss at 6 or 12 months following surgery. Approximately 3–8 % of patients with GJ strictures, most with late strictures, require more than three dilations for resolution [17]. These patients may benefit from placement of a feeding tube in the gastric remnant to allow for caloric supplementation [19].
Perforation is the greatest concern after endoscopic dilatation. Large series published on post-RYGB GJ stricture dilatation have demonstrated a 0.6–2.2 % perforation rate [16]. To minimize the risk of perforation, some surgeons will not dilate a stricture more than 3 mm (or 9 Fr) during a single session. However, studies have not demonstrated an increased risk of perforation due to balloon size or number of dilations [14]. Some surgeons also believe that the more rigid Savary-Gilliard bougies offer a better, more durable dilation than the more pliable pneumatic balloons and should be used following initial balloon dilation [19]. Intralesional steroid injections have been reported for cases of refractory strictures. Steroids are thought to prevent cross-linking of collagen and thus prevent fibrotic healing. However, the role of steroid injections is still not defined as some studies have found that they provide no added benefit [14]. For patients with GJ strictures refractory to endoscopic therapy, operative revision of the anastomosis can be very effective, with a success rate of more than 95 % [13]. However, these revisional procedures are often technically challenging and complex. Most refractory anastomotic strictures are thought to be due to large-volume gastric pouches, which result in excessive acid production. These situations ultimately require major downsizing of the proximal gastric pouch to less than 10 mL in volume to help ensure that the pouch contains only gastric cardia and excludes all acid-producing gastric mucosa [13].
12.2.2 Jejunojejunal Stricture
Jejunojejunal (JJ) stricture is one of the leading causes of SBO in the early postoperative period, with an incidence of 0.4–1.2 % and a mean interval to presentation of 10–15 days [2, 3, 9]. Jejunojejunal narrowing usually occurs in the Roux limb portion of the anastomosis because of technical error when too much tissue is taken using a double-stapling technique with a linear stapler [2]. A large study demonstrated that stapled closure of the common enterotomy resulted in a significantly higher rate of JJ obstruction compared with hand-sewn closure [4]. Brolin also reported that bowel obstruction can occur at the afferent limb of the jejunojejunostomy after open RYGB and advocated placement of an “anti-obstruction suture” to prevent kinking at the level of the anastomosis [22]. The majority of JJ strictures present with nonspecific clinical symptoms and signs suggestive of partial SBO. However, these types of strictures can be easily diagnosed with UGI series or CT imaging [2]. Postoperative edema can also cause early JJ obstruction that tends to be partial and responds well with conservative management [9]. Narrowing of the jejunojejunostomy due to incorrect stapling may ultimately require creation of a new side-to-side anastomosis proximal to the obstruction site [11].
12.2.3 Intussusception
Retrograde intussusception post-RYGB is usually located at the jejunojejunostomy, progressing from distally to proximally (i.e., antiperistaltic), rather than the more common proximal to distal direction (i.e., isoperistaltic) [23]. This postoperative complication is quite rare, with a reported incidence of less than 0.6 % in bypass patients [24].
Intussusception usually occurs after significant weight loss and its cause appears to be multifactorial. Most reports of retrograde intussusception have described an absence of any definable lead point [25]. However, staple lines, sutures, and adhesions have been proposed as possible lead points [26].
Although the exact mechanism is not yet clear, motility disturbances are believed to be the most likely cause of intussusception after RYGB [24]. In RYGB, the distal jejunum is separated from the duodenal pacemaker and disrupts the propagation of the natural pacemaker into the Roux limb [24]. As a result, ectopic pacemakers arise in the Roux limb, which can generate pacesetter potentials in both distal and proximal directions [24]. Manometric studies have confirmed that patients after RYGB have a high incidence of motility disorders secondary to these ectopic pacemakers [27]. It is hypothesized that an ectopic pacemaker can create a peristaltic contractile wave that reaches the jejunojejunostomy at the same time as a normal peristaltic wave from the duodenum, producing a high-amplitude peristaltic wave in the proximal channel that engulfs the bowel distal to it [27]. Motility disorders can also contribute to “Roux stasis syndrome,” a condition characterized by chronic abdominal pain, nausea, and vomiting [24].
The clinical presentation of intussusception can vary, ranging from chronic intermittent abdominal pain to sudden severe intractable pain consistent with complete obstruction and bowel ischemia [23]. The most common presentation is vague abdominal pain (100 %) followed by vomiting (40 %) and bloody stools (20 %) [26]. This type of complication appears to present more commonly late after surgery and in those patients with substantial weight loss [28].
Plain abdominal films are usually unreliable in the evaluation of intussusception [25]. Contrast-enhanced CT is diagnostic in most cases, and a characteristic “target sign” at the site of the intussusception is usually pathognomonic [23]. CT findings also include a dilated gastric remnant consistent with an obstructed biliopancreatic limb [28].
Patients with evidence of intussusception require immediate surgery to rule out bowel ischemia. Therefore, there should be a low threshold for laparoscopic exploration in suspected cases. The options for surgical management include reduction alone, reduction with enteropexy, and resection of the JJ with reconstruction of the anastomosis [28]. Simper et al. found a 100 % recurrence rate associated with reduction alone [23]. However, Varban et al. demonstrated that reduction with or without enteropexy could achieve equivalent morbidity and low recurrence compared with resection [28]. Most authors would recommend an en bloc resection of the affected segment and reconstruction of the anastomosis given the high prevalence of bowel infarction and risk of perforation [25, 26].
12.2.4 Bezoar
12.2.4.1 Hemobezoar (Intraluminal Blood Clot)
Acute postoperative bleeding after LRYGB is estimated to occur in approximately 3 % of patients [29]. Accordingly, obstruction due to an intraluminal clot is exceedingly rare [4]. Intraluminal bleeding is usually self-limiting and likely from the GJ anastomosis or from the staple lines of the gastric remnant. The JJ can also bleed or act as a locus for clot formation from the passing blood [30]. Staple line bleeding in laparoscopic RYGB has been shown to be three times more likely than in open RYGB. Although bleeding from anastomotic staple lines may be appreciated during surgery, the lumen of the gastric remnant cannot be visualized after the pouch is constructed.
The most common symptom in this type of obstruction is a sense of impending doom, which is likely associated with acute gastric remnant dilation. Tachycardia is reported to be the most common sign in these patients. As with any early postoperative acute mechanical SBO, immediate treatment to avoid serious complications is required.
Placement of a gastrostomy tube is necessary to decompress the dilated gastric remnant and to permit enteral access in the postoperative period. Intraoperative endoscopy to suction the clot may be difficult given the distance required to reach the anastomosis. Endoscopic insufflation may also further dilate the bowel and lead to perforation. An enterotomy to evacuate the blood clot may be useful if the common channel appears to be completely collapsed. Anastomotic revision may be necessary if the anastomosis appears to be disrupted from the obstruction [11].
12.2.4.2 Phytobezoar
Phytobezoars are retained concretions of undigested fruit or vegetable fibers in the GI tract [31]. These are the most common foreign body of the gastrointestinal tract with SBO being the most commonly associated complication [32]. Nonetheless, SBO due to bezoar formation after RYGB is rather uncommon. Management can include chemical dissolution (i.e., cellulase), endoscopy (i.e., fragmentation and flushing), or surgical evacuation [31]. Complete impaction may require an enterotomy to remove the impacted phytobezoar, which can be done laparoscopically. Bowel resection is rarely indicated in these situations [32]. It is important to provide patients with appropriate nutritional counseling and/or psychiatric evaluation in the postoperative setting to help avoid future recurrences.
12.3 Extraluminal Obstructions
12.3.1 Incarcerated Ventral Hernia
Obesity, or more specifically central adiposity, is associated with an increased risk of umbilical and incisional hernia [33]. Although many bariatric surgeons have reported their experience with bowel obstruction from incarcerated abdominal wall hernias, there is still no consensus as to the optimal treatment of ventral hernia in bypass candidates. Ventral hernia in patients presenting for RYGB surgery has posed a therapeutic dilemma for two main reasons: the high recurrence rate after primary repair (~50 %) and the potential of mesh infection from contamination during surgery [33].
Umbilical hernias smaller than 3–4 cm in diameter can be closed primarily at the end of an RYGB using transabdominal sutures but can still have a recurrence rate of over 20 % [33]. If omentum is present in the sac, it should not be reduced. Instead, a rent can be made between the hernia and transverse colon to allow sufficient access to run the small bowel. If a hernia is found completely reduced, patients have an increased risk of developing SBO postoperatively. Some surgeons would advocate performing a sleeve gastrectomy over an RYGB in this setting, to avoid the potentially devastating complications associated with obstructed bypass patients. There is evidence that concomitant repair of ventral hernias with biological mesh can be a safe and effective alternative in these cases [33].
In a series reported by Cho et al. where closure of port-site abdominal fascia was not routine, the hernia incidence was 0.14 % [2]. Larger trocar size and cutting trocars have been associated with the development of port-site hernias [4]. Fortunately, dilating trocars have decreased the need for fascial closure of trocar sites <12 mm in diameter [4]. In a review by Koppman et al., port-site herniation resulted in bowel obstruction in 0.3 % of patients [4]. Hernias within the preperitoneal spaces have been reported, prompting some to suggest that peritoneal closure should be incorporated during port-site closure. A full-thickness closure can be performed safely using port-site closing devices [11].
12.3.2 Internal Hernia
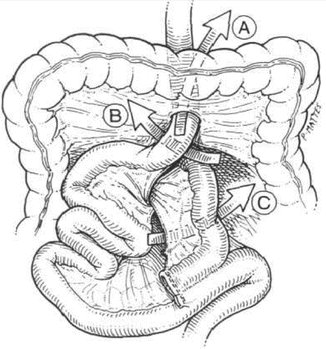
A full discussion of internal hernia is provided in Chapter 11. However, a brief overview of this pathology as it leads to bowel obstruction is also included here. The incidence of internal hernia after LRYGB had been reported to be 1–4 % [12, 34, 35]. Antecolic, antegastric LRYGB approach significantly reduces the incidence of internal hernia compared to the retrocolic, retrogastric approach because it eliminates the mesocolic defect [1, 2]. The predisposing factor for the development of internal hernia is that mesenteric fat is lost quickly with weight reduction and enlarges the surgically created mesenteric defects [11]. As well, the mesentery can end up tearing or loosening at the level of the sutures in cases where the defects have been closed [34]. There are several possible defects that can lead to internal herniation (Fig. 12.2).