Fig. 10.1
Sites of potential internal hernia defects following LRYGB, including the mesocolic window or retrocolic tunnel (green arrow), Petersen’s defect (blue arrow), and mesomesenteric or distal anastomosis defect (red arrow). With kind permission from Comeau E, Gagner M, Inabnet WB, Herron DM, Quinn TM, Pomp A. Symptomatic internal hernias after laparoscopic bariatric surgery. Surg Endosc. 2005;19:34–9 [38]. © Springer
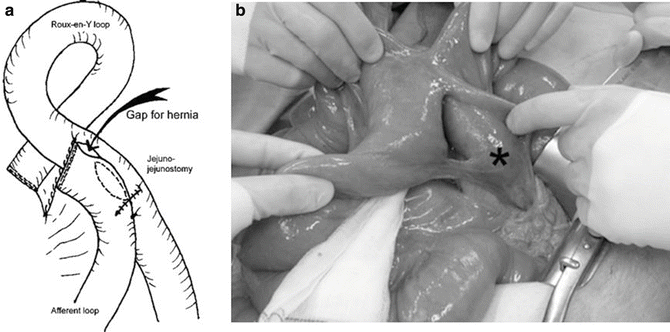
Fig. 10.2
(a) Schematic drawing of the jejunojejunostomy showing the location of a new type of internal hernia reported by Paroz et al. (b) Intraoperative photograph demonstrating the gap that has developed between the two jejunal loops. The asterisk denotes the end of the biliopancreatic limb. This type of internal hernia does not involve a mesenteric defect. With permission from Paroz A, Calmes JM, Romy S, Giusti V, Suter M. A new type of internal hernia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2009;19:527–30 [16]. © Springer
10.2 Prevention
An internal hernia can be a devastating complication of LRYGB, and there has been considerable interest in operative techniques to minimize their occurrence. The two major areas of debate are antecolic vs. retrocolic positioning of the Roux limb and closure vs. nonclosure of the mesenteric defects. Regarding positioning of the Roux limb, most studies support bringing the Roux limb anterior to the transverse colon [6, 17, 18]. This has the obvious advantage of eliminating one of the potential sites of internal hernia, the mesocolic defect. Koppman et al. combined data of all LRYGB cases performed at their institution with those identified in a Medline search of the published literature to review small bowel obstruction after LRYGB in 9527 patients [6]. The overall incidence of small bowel obstruction was 3.6 % and internal hernia was the most common cause accounting for 42 % of the obstructions. When data were stratified according to position of the Roux limb, the rate of internal hernia was significantly higher after retrocolic vs. antecolic placement (2.4 % vs. 0.3 %, respectively; p < 0.0001). A study by Escalona et al. also demonstrated a significantly higher internal hernia rate with the retrocolic vs. antecolic technique (9.3 % vs. 1.8 %, respectively; p < 0.001), and the retrocolic position was identified as a risk factor for internal hernia on multivariate analysis (p < 0.001) [18]. Advocates of the retrocolic approach have suggested that careful defect closure may result in a decreased internal hernia rate [19]. However, it is notable that all of the mesenteric defects were closed in both the retro- and ante-colic technique in the Escalona et al. study, yet there was still a fivefold decrease in internal hernia rate with antecolic positioning of the Roux limb [18]. Thus, overall the literature favors an antecolic gastric bypass with a retrocolic Roux limb being acceptable in patients whose anatomy does not allow the creation of a tension-free antecolic Roux limb [6, 19].
Current studies also favor the complete closure of all mesenteric defects created during LRYGB [9, 11, 12, 20–22]. Four studies comparing nonclosure with closure of mesenteric defects reported a significantly decreased number or incidence of internal hernias after closure [11, 20–22]. Iannelli et al. described an overall internal hernia incidence of 1.6 % [20]. When stratified by nonclosure or closure of mesenteric defects, there was a decrease in the rate of internal hernia from 1.9 to 0.6 %, respectively [20]. A second study from de la Cruz-Muñoz et al. demonstrated an overall incidence of 1.8 %, and a greater number of patients developed internal hernia with nonclosure of the mesenteric defect at the jejunojejunostomy (p < 0.001) [21]. However, the authors did not report the denominator for the number of patients in each group, so that the incidence for each group could not be calculated. Brolin and Kella saw a decrease in internal hernia rate from 2.6 to 0.5 % after changing their practice to closure of the mesenteric defect (p = 0.056) [22]. Although the studies by de la Cruz-Muñoz et al. and Brolin and Kella support the closure of the mesomesenteric defect alone, other studies have reported significant rates of internal hernia at the mesocolic and Petersen’s defects [10, 11]. Bauman et al. examined 1047 patients in their practice and found an internal hernia rate of 6.2 % at Peterson’s space and 0.7 % at the mesomesenteric site [11]. The rate of internal hernia at Peterson’s space decreased to 0 % after changing their practice to closure of this defect. Although several techniques have been described, the most common technique for closure of potential hernia sites is a running nonabsorbable suture, in either simple or purse-string fashion [9, 10, 23, 24]. Those who oppose closure of the defects argue that improper closure may cause tension on the anastomosis, hematomas, or injury to the mesenteric blood vessels [19]. This highlights the importance of taking care to close defects using only superficial closely spaced sutures of the mesentery to avoid injury. Given the data presented above, it must be noted that internal hernias still occur in patients who have their mesenteric defects closed but at a lower incidence. This may be due to improper closure, incomplete closure from tearing of the mesentery, or reduction in intra-abdominal fat as significant weight loss occurs, allowing the hernia spaces to expand [20].
As mentioned previously, it is difficult to know the true incidence of internal hernia with any technique, since patients may be lost to follow-up and there are different follow-up intervals between compared groups. For both antecolic vs. retrocolic positioning of the Roux limb and closure vs. nonclosure of mesenteric defects, the data cited have compared a change in technique from an earlier to later practice. This results in an inherently shorter follow-up interval for antecolic and closure of potential hernia sites groups. The Koppman et al. study reviewed papers with a range of overall follow-up from 4 to 43 months, and follow-up interval was not reported in 7 of 17 studies included [6]. The Escalona et al. study notes a median follow-up of 16 months. Neither report what the follow-up interval was for the antecolic vs. retrocolic groups separately. Similarly, the follow-up interval for patients with nonclosure vs. closure of mesenteric defects was not reported in the Iannelli et al. or de la Cruz-Muñoz et al. studies [20, 21]. The de la Cruz-Muñoz et al. group did indicate the percentage of patients at 1- and 5-year follow-up was 62 % and 60 % vs. 37 % and 30 % with nonclosure or closure, respectively [21]. The Brolin and Kella study reported a mean follow-up of 100 ± 12 months vs. 40 ± 14 months for nonclosure vs. closure groups, respectively [22]. Thus, the lower internal hernia rate in these studies could in part be attributed to the shorter follow-up interval for patients in the antecolic and closure of mesenteric defects groups. However, most literature supports the interval from LRYGB to development of internal hernia to be less than 1–3 years [4, 9, 10, 20, 22].
A recent study at our institution demonstrated a significant decrease in the rate of internal hernias with antecolic positioning of the Roux limb and closure of the mesenteric defects [4]. Like many practices, ours evolved from retrocolic positioning and nonclosure to an antecolic Roux limb and closure of both mesenteric defects. Our internal hernia rate decreased from 8.4 to 3.8 % (p = 0.005). Median length of overall follow-up was 56 months (range, 13–113). When stratified by technique, median follow-up for the nonclosure of defects group was 73 months (range 17–113) and 41 months (range 13–90) for the closure group (p = 0.001) [4]. Overall median time to develop an internal hernia was 22.6 months (range 3–103) months, and it was longer in the nonclosure group [33.5 months (range 10–103) vs. 16.6 months (range 3–72), respectively; p < 0.001] supporting that we are capturing more internal hernias with longer follow-up intervals. Whether a decreased internal hernia rate using the antecolic positioning of the Roux limb with closure of defects technique would persist given a comparable length of follow-up remains to be determined.
Other surgical techniques have been proposed to decrease the incidence of internal hernia. In their study, Quebbeman and Dallal changed the orientation of the end of the Roux limb so that it faced the greater curvature of the stomach and the rate of internal hernia decreased from 9 to 0.5 % [25]. The authors theorized that the decrease in internal hernias was due to the Roux limb mesentery lying on the right side of the ligament of Treitz, with better apposition of the two mesenteries. Nandipati et al. demonstrated that rotating the Roux limb counterclockwise allowed the jejunojejunostomy to be located on the left side of the abdomen, allowing the jejunojejunostomy to lie in its more natural position on the left side of the mesenteric axis [26]. The overall internal hernia rate was 4.7 %. When stratified by rotation of the Roux limb, there was a significant decrease in the incidence of internal hernias with counterclockwise vs. clockwise rotation (0.7 % vs. 6.9 %, respectively; p = 0.0018). According to the authors, counterclockwise rotation also makes the mesenteric defect easier to close completely. Other methods described include minimal division or nondivision of the small bowel mesentery, division of the omentum with tucking the bisected omentum to each side of the Roux limb, creation of a long jejunojejunostomy, placement of the jejunojejunostomy above the colon in the left upper quadrant, and a shorter biliopancreatic limb [11, 13, 17, 20, 27].
Because an internal hernia is a potentially devastating complication, we recommend using the strategies discussed above to minimize the occurrence. In our practice, we implement the following techniques: (a) antecolic antegastric positioning of the Roux limb, (b) counterclockwise rotation of the alimentary limb, (c) nondivision of the small bowel mesentery unless necessary, (d) orientation of the stapled end of the Roux limb toward the left upper quadrant, (e) omental division and placement on each side of the Roux limb, (f) a 40-cm biliopancreatic limb, and (g) routine closure of both Petersen’s and mesomesenteric defects with a running nonabsorbable suture (Figs. 10.3 and 10.4).
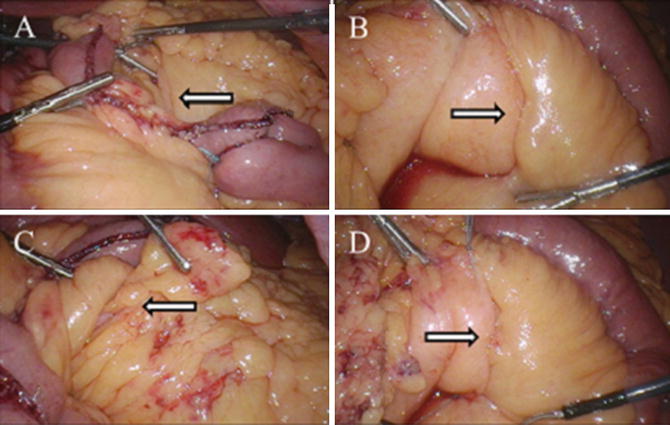
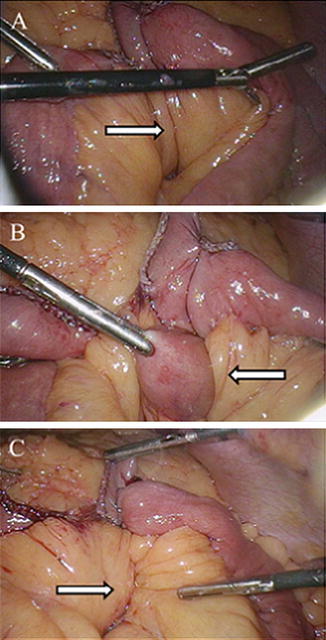

Fig. 10.3
Intraoperative photos during LRYGB demonstrating (a) the leaflets of mesocolon and Roux limb mesentery (Petersen’s defect) as seen from the left side of the patient (arrow), (b) Petersen’s defect as viewed from the right side of the patient (arrow), (c) Peterson’s space as viewed from the left with arrow demonstrating fat and small bowel attempting to herniate through the defect (arrow), (d) closure of Petersen’s defect from the patient’s right side with running, nonabsorbable suture (arrow). Photos kindly provided by Dr. Richard Stahl

Fig. 10.4
Intraoperative photos during LRYGB demonstrating (a) the mesenteric defect created at the jejunojejunal anastomosis (arrow), (b) bowel herniating through this site (arrow), and (c) closure of the mesomesenteric defect with running, nonabsorbable suture (arrow). Photos kindly provided by Dr. Richard Stahl
10.3 Diagnosis
Diagnosing an internal hernia in a patient after LRYGB can be challenging, since many patients with a symptomatic internal hernia have nonspecific complaints of abdominal pain, nausea, and vomiting [4, 9, 11]. The clinical history of the patient often contributes to the differential diagnosis. While internal hernias may occur at any time following an operation, patients who have recently undergone their operation are more likely to suffer from an anastomotic leak or adhesive disease [28]. Anastomotic strictures may also present with postprandial fullness, nausea and vomiting, and abdominal pain [6]. Patients who have greater weight loss and are at least 1–3 years from LRYGB are thought to be at highest risk for internal hernia. Greater weight loss is thought to result in enlargement of mesenteric defects due to loss of intraperitoneal fat, thereby increasing the risk of internal hernia [28]. Abdominal examination and laboratory evaluation may be unrevealing as well [9, 29]. Imaging can be very helpful in diagnosing an internal hernia and is best performed when the patient is having symptoms, since some internal hernias may spontaneously reduce and recur, leading to intermittent pain [30]. It is crucial to remember that imaging may be negative in 20 % of patients [4, 9].
Findings consistent with the presence of internal hernia on upper GI series and CT imaging have been described in Roux-en-Y gastric bypass patients [28, 31]. In the Blachar et al. study, there was considerable overlap in a comparison of findings on upper GI series in patients with adhesions vs. internal hernia as the cause of small bowel obstruction, leading the authors to conclude that a specific cause of small bowel obstruction could not be made using this modality [28]. Findings of small bowel obstruction and distended small bowel segments > 2.5 cm were both present in 100 % of patients with adhesions vs. internal hernia. A diagnosis of internal hernia was favored with the finding of a cluster of dilated loops of small bowel located in the left upper or middle abdomen, which remained high in the abdomen with the patient in erect position. Ahmed et al. found that upper GI series had a positive finding suggestive of internal hernia in 65 % of their patients [31]. The four most recurring findings were dilated fluid-filled loops of small bowel, redundant Roux limb in the lesser sac, a preponderance of small bowel loops in the left upper quadrant, and slow emptying of contrast with prolonged transit times.
CT imaging has emerged as the preferred imaging modality in gastric bypass patients who are having symptoms of small bowel obstruction. There are many reasons for this. First, CT imaging typically has less technical difficulties than may be encountered when performing an upper GI series on a patient with obesity, such as difficult positioning or poor image quality [31, 32]. CT imaging often provides more rapid diagnostic information in the acute setting, and it is more widely available since some facilities may not have qualified staff available to perform upper GI series at night and on weekends. Additionally, upper GI series is a dynamic study. Review and interpretation by the surgeon is dependent on the images captured by the radiology team. In contrast, CT images are more readily interpreted by surgeons, and review by a bariatric surgeon may improve the diagnostic yield [33]. Finally, CT imaging is more sensitive and specific than other reported imaging techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







