Antoine E. Khoury, MD, FRCSC, FAAP, Darius J. Bägli, MDCM, FRCSC, FAAP, FACS VUR has a unique distinction of having evolved from an anatomic curiosity around the first century AD into one of the most contentious and complex areas of urology today. Galen and da Vinci made the first references to VUR by Western Medicine when they alluded to the ureterovesical junction (UVJ) as a mediator of unidirectional flow of urine from the kidneys to the bladder. Although VUR was first demonstrated to be a normal finding in dogs and rabbits (Semblinow, 1907), it was a gynecologic misadventure. It revealed that VUR in human adults may not be a normal state (Pozzi, 1893). Sampson, in 1907, suggested that the oblique course of the ureter through the bladder wall created a locking mechanism at the UVJ and was also the first to imply that VUR might lead to renal infection. Although it was not possible to uniformly demonstrate VUR in all people in cadaveric studies (Young, 1989), it was not until the UJV had been more clearly dissected that it was realized the incidence of VUR varied with the length of the ureter’s obliquity in the bladder and the formation of the trigone (Gruber, 1929). Pivotal discoveries occurred when Hutch (1952) reported on a relationship between VUR and chronic pyelonephritis in paraplegic patients and Hodson (1959) observed that urinary tract infection (UTI) and renal scarring carried a high likelihood of VUR in children. Two seminal bodies of work defined the modern era of VUR. The first, the work of Ransley and Risdon (1979), defined the pathophysiology of reflux nephropathy by demonstrating the relationship among infection, reflux, and pyelonephritic scarring. Secondly, these observations complemented the clinical studies of Smellie (1991) and colleagues, who galvanized the related concepts inherent in clinical UTI, bacterial pyelonephritis, renal scarring, and VUR. In 1985 a consensus system for the grading of reflux was published by Lebowitz and colleagues (1985) on the basis of the International Reflux Study Group’s deliberations. The tendency for VUR to resolve spontaneously on the one hand or persist beyond its natural resolution rate due to abnormal bladder dynamics makes it difficult to confidently generalize the true prevalence of reflux from a given population. In one meta-analysis of studies of children undergoing cystography for various indications (Sargent, 2000), the prevalence of reflux was estimated to be approximately 30% for children with UTI and 17% without infection. In contrast, reflux may be present in up to 70% of infants who present with UTI (Baker et al, 1966). In a population of 157 adults investigated for incidental hypertension without any evidence of renal abnormality, the prevalence of VUR was estimated to be 19%, with high-grade reflux in more than half of the refluxing group (Barai et al, 2004). Reflux is relatively uncommon in adult males (Chapple et al, 1990). In asymptomatic infants followed up for antenatal hydronephrosis, the prevalence of reflux ranges from 15% in infants with absent or mild hydronephrosis on postnatal ultrasound (Phan et al, 2003) to 38% in a group of neonates with various postnatal upper tract sonographic anomalies including hydronephrosis, renal cysts, or renal agenesis (Zerin et al, 1993). Differences in reflux rates between males and females may suggest a sexual dichotomy in the function of the lower urinary tract, bladder outlet, and urethra. In one study of 117 infants assessed for reflux following fetal upper tract dilation, 76% of refluxing infants were male (Ring et al, 1993). In later life, the likelihood of having reflux if presenting with a UTI is higher if male than female (Shopfner, 1970), even though the great majority (85%) of prevailing reflux in older children is in females. A confounding factor in understanding true gender differences in reflux between males and females is the gender-driven predisposition to UTI. Uncircumcised male infants show a 12-fold greater risk for UTI than circumcised males, as well as a greater propensity for harboring periurethral uropathogenic flora (Wiswell et al, 1988; Wiswell and Hachey, 1993). The greater incidence of UTI will necessarily invite more frequent evaluation and therefore detection of VUR in this population. It is not known whether the incidence of reflux detection would rise in females if they were to be incidentally evaluated for reflux as often as infant males. In a related finding, only 10% of patients entered into the International Reflux Study in Children from the United States were boys compared with 24% entered from Europe. The circumcision rate in the latter group was only 5% compared with 62% in the U.S. group (P < .001) (Weiss et al, 1992b). Most studies of fetal reflux do not in fact detect reflux per se in the fetus but relate fetal hydronephrosis parameters to reflux in the neonatal period. However, fetal hydronephrosis is common, often resolves, and has low specificity for postnatal VUR. Nevertheless, fetal hydronephrosis is commonly associated with postnatally detected reflux. Zerin described a 38% detection rate of reflux in 130 neonates with prenatal hydronephrosis (Zerin et al, 1993). In 19% the reflux was bilateral. It is suggested that the lower the threshold for defining hydronephrosis (in millimeters of pelvic diameter) in the fetus, the more often reflux will be detected postnatally (Anderson et al, 1997). This leads to speculation as to whether reflux is a normal variant in the population but becomes clinically relevant only in some because of a predisposition to UTI, a conclusion supported by the observation that reflux without infection is of questionable clinical significance. Boys appear to harbor postnatal reflux more commonly—a 6 : 1 male-to-female ratio was reported in one study of 27 cases (Marra et al, 1994). The highest grades of reflux are most commonly associated with renal scintigraphic abnormalities. In many cases, even in the absence of any infection history from birth, the presence of a small kidney with globally reduced scintigraphic function may indicate that such renal scintigraphic abnormalities may be associated with a developmental ureteral bud abnormality associated with high-grade reflux or secondary to the reflux itself (Oliveira et al, 1998; Stock et al, 1998). As stated previously, because the natural history of reflux involves spontaneous resolution over time, it is self-evident that less primary reflux would be prevalent in older children compared with infants (Table 122–1). Even in the presence of infection or asymptomatic bacteriuria, reflux is more common in younger patients (Smellie, 1991). Table 122–1 Incidence of Reflux in Patients with Urinary Tract Infections From Baker R, Maxted W, Maylath J, et al. Relation of age, sex, and infection to reflux: data indicating high spontaneous cure rate in pediatric patients. J Urol 1966;95:27. Little is known about racial predisposition to reflux worldwide because reflux studies have generally been restricted to western countries. One difference established over several studies is the relative 10-fold lower frequency of reflux in female children of African descent (Skoog and Belman, 1991; Chand et al, 2003). In addition, reflux resolved sooner in this population (P < .005). In a series of non-Caucasian children with a 4 : 1 male-to-female ratio, 58% of whom were younger than 1 year of age presenting with UTI (72%), voiding difficulties (10%), or other malformations (14%), reflux was present in only 10% (West and Venugopal, 1993). Even in follow-up of antinatal hydronephrosis, reflux was found in 17.6% of 51 nonblack versus 0% of 58 black infants (Horowitz et al, 1999). Such differences may involve a delay in maturation of the antireflux mechanism in Caucasian patients as the race-associated frequency of reflux becomes equal regardless of race after 10 years of age (Melhem and Harpen, 1997). A recent meta-analysis of sibling reflux studies suggests the prevalence of VUR in siblings to be approximately 32% (Hollowell and Greenfeld, 2002). However, the prevalence may be as low as 7% in older siblings (Connolly et al, 1996) or as high as 100% in identical twin siblings (Kaefer et al, 2000). The latter finding undeniably supports the notion that VUR can be an inherited condition and that the genetic mode of transmission may be autosomal dominant. Although a heightened prevalence of reflux exists in siblings of refluxing index patients, the natural history of reflux would suggest that siblings who are older harbor reflux less often compared with relatively younger siblings because of spontaneous reflux resolution (Connolly et al, 1996). However, none of the existing sibling studies rigorously state whether the prevalence of sibling reflux is dependent on whether the sibling is younger or older than the index patient herself. By virtue of its detection by screening, sibling reflux is usually asymptomatic at the time of diagnosis. Furthermore, the tendency for reflux to have resolved before any renal changes such as focal scarring detected by imaging can be reliably ascribed to the reflux itself further complicates the management of reflux in siblings. These clinical features underscore the difficulties inherent in formulating meaningful recommendations for the management of sibling reflux detected by screening. In one retrospective study of 123 screened siblings, 44 (36%) demonstrated VUR on voiding cystography (Houle et al, 2004). Thirty-seven of these patients underwent renal imaging. Ultrasound was abnormal in 30% and renal scintigraphy, when used, was abnormal in 28%. However, in siblings older than 2 years, renal scintigraphic abnormalities were twice as common as in the entire group of siblings. The authors concluded that renal damage is therefore progressive in the older siblings and proposed earlier screening of siblings of refluxing index patients. However, this study fails to address the fact that renal damage or aberrant formation may have occurred very early and over time (beyond 2 years of age), renal growth may have exaggerated the appearance of such scintigraphic abnormalities. Scintigraphic results may become exaggerated by normal cortical growth surrounding a scarred region that has failed to grow or by compensatory hypertrophy of a contralateral kidney. The missing link is a prospective scintigraphic or sonographic follow-up of asymptomatic refluxing infant sibling children from birth onward. Finally, the reporting of scintigraphic results by many sibling reflux studies is confounded by failing to differentiate between aberrant renal development often associated with higher grades of reflux and true scarring secondary to infection and inflammation. Moreover, the ability to modulate the course of the processes that mediate congenital dysplasia is not possible presently, as it is with scarring. In the absence of such data and faced with the invasive nature of the gold standard test for reflux, the cystogram, one is left with the following questions. In addition to sibling reflux, a prospective screen of progeny of refluxing patients revealed a 66% rate of reflux in the offspring (Noe et al, 1992), which further strengthens the notion of an autosomal dominant component to the genetic mechanism of reflux. The substantially higher rate of reflux in siblings and progeny of index patients than in the general population defines these patients as susceptible to renal morbidity. Segregation and linkage analyses have implicated a number of loci in the pathogenesis of VUR, though no specific gene product or functional role for these loci in reflux has yet been identified (Chapman et al, 1985; Feather et al, 2000). Several studies have used a morphogenetic approach to search for candidate genes, which may underlie VUR. The original ureteral budding studies of Mackie and Stephens (1975), which correlate the position of ureteral budding from the mesonephric (wolffian) duct with that of the final ureteral orifice (UO), provide a modern basis for a genetic interpretation of VUR. Several genes have been observed to regulate these developmental processes and, by extension, are believed to serve as potential regulators of ureterovesical junction integrity. Indeed, genetic misregulation of ureteral budding is believed to underlie many congenital anomalies of the kidney and urinary tract (often referred to as CAKUT) (Miyazaki and Ichikawa, 2003). PAX2 is a transcription factor regulating kidney, central nervous system, and ocular development in mice. It is necessary for ureteric budding in mice (Keller et al, 1994). PAX2 is located on human chromosome 10q, and mutations have been reported in human syndromes involving colobomas and renal anomalies including hypoplasia, dysplasia, glomerulonephritis, and VUR (Sanyanusin et al, 1995). However, PAX2 has not been shown to be a major determinant of primary VUR (Choi et al, 1998; Cunliffe et al, 1998). Glial-derived neurotrophic factor (Gdnf) and its receptor RET show strong involvement in ureterovesical junction formation in mice (Yu et al, 2004). Overexpression of RET in mice leads to abnormal placement of the ureteral bud and is associated with a 30% incidence of VUR at birth compared with 4% in wild-type mice. Nevertheless, the Gdnf RET signaling complex has not been found to mediate VUR in humans (Shefelbine et al, 1998). Another fascinating animal model of VUR is observed following depletion of the uroplakin III gene (Hu et al, 2000). However, no structural uroplakin III gene alterations have been noted in human cohorts with VUR (Giltay et al, 2004; Jiang et al, 2004). One explanation for this current finding may be that major uroplakin mutations are developmentally lethal in humans. Finally, members of the renin-angiotensin family of proteins have been implicated in several renal and ureteral developmental anomalies including ureteropelvic junction (UPJ) obstruction and megaureter (Hohenfellner et al, 1999). Although associations between angiotensin receptor-2 (Agtr2) (Yoneda et al, 2002) and angiotensin-converting enzyme (ACE) (Liu et al, 2004) genes with VUR have been sought, no definitive etiologic link with VUR has been found. Notwithstanding an observed pattern of autosomal dominant inheritance in some families, the failure to identify any strong genetic mechanism in primary VUR in humans despite the presence of convincing animal genetic models of reflux argues for a more complex polygenetic mechanism of disease in humans. A full discussion of the embryology of the trigone and ureteric orifice is found elsewhere in the text. In brief, two events proceed simultaneously to govern the ultimate position and integrity of the UVJ. At one point, the embryonic ureter buds from the mesonephric or wolffian duct to define the metanephric duct or early fetal ureter. The wolffian duct (early vas deferens) and early ureter can be thought of as forming the two upper arms of a Y with the distal mesonephric duct as the stem of the Y. While budding is occurring, the distal mesonephric duct is being drawn and incorporated into the region of the urogenital sinus, which later becomes the bladder. Incorporation continues until the entire stem is absorbed, leaving the two arms of the Y to enter the bladder separately: one as the ureter and the other as the vas and ejaculatory duct in the male prostatic urethra (or the vestigial Gartner duct in the female vagina). The two arms of the Y also rotate relative to each other once they contact the urogenital sinus (UGS)/bladder wall resulting in the UO being proximal to the ejaculatory duct orifice. If the ureteral bud reaches the UGS too soon (believed to be due to early budding), over-rotation draws it high and lateral in the bladder wall, leading to inadequate incorporation, insufficient intramural length in the bladder wall, and reflux (Mackie et al, 1975). If the ureteral bud reaches the UGS too late (due to budding late), insufficient rotation occurs, resulting in an ectopic ureter that is drawn distally and medially, often obstructing in the bladder neck region or elsewhere. Furthermore, early or late budding is also thought to mistarget the contact between bud epithelium and the metanephros, leading to renal malformations, dysplasia, hypoplasia, or even agenesis. First, for purposes of reflux prevention, the ureter represents a dynamic conduit, which adequately propels the urine presented to it in a bolus fashion, antegrade, by neuromuscular propagation of peristaltic activity. In so doing, reflux is actively opposed. Moreover, if reflux were to occur, depending on its degree and timing, antegrade flow might be expected to keep refluxing urine from reaching the renal pelvis. The second component is the anatomic design of the UVJ. At the heart of this unique mechanism lies an intramural portion of ureter that travels within the detrusor muscle as it traverses the bladder wall (Fig. 122–1) (Elbadawi, 1972). At the extravesical bladder hiatus, the three muscle layers of the ureter separate. The outer ureteral muscle merges with the outer detrusor muscle to form Waldeyer sheath. The latter contributes to formation of the deep trigone. The intramural ureter remains passively compressed by the bladder wall during bladder filling, preventing urine from entering the ureter. Adequate intramural length and fixation of the ureter between its extravesical and intravesical points is required to create this antirefluxing compression valve. Paquin’s early dissections of the UVJ in children revealed an approximately 5 : 1 ratio of tunnel length to ureteral diameter in nonrefluxing junctions compared with a 1.4 : 1 ratio in refluxing UVJs (Paquin, 1959) (Table 122–2). Intravesically, the inner muscle of the ureter merges with detrusor muscle to contribute to the superficial trigone. Some of these inner ureteral fibers pass medially to contribute to the intraureteric ridge (Mercier bar). The cellular and molecular details that characterize normal and refluxing UVJs are still unknown. However, it is likely that in addition to architectural deficiencies of tunnel length, abnormalities in UV smooth muscle and extracellular matrix composition and neural function may contribute to reflux (Oswald et al, 2004). (A, From Glenn J, editor. Urologic surgery. 2nd ed. New York: Harper & Row; 1975; B, from Elbadawi A. Anatomy and function of the urethral sheath. J Urol 1972;107:224.) Opening of the UVJ is achieved by active contraction of the longitudinal muscles within the tunnel. This draws the extravesical and intravesical points of the intramural ureter closer together, shortening and widening the tunnel, and allows passage of the urine bolus into the bladder. Indeed, when viewed cystoscopically, a lateral displacement of the UO accompanies the classic jet of urine into the bladder. Although such lateral displacement is functionally normal and necessary to permit urine to pass, permanent lateral displacement by virtue of a constitutively short tunnel characterizes the cystoscopic position of the refluxing UO. Closure of the UVJ results both from compression of the intramural ureter and a return to its full tunnel length as the ureteral muscle relaxes. Thus active and passive mechanisms dynamically reconfigure the tunnel as needed to allow antegrade passage of urine while preventing retrograde flow. Finally, the existence of local efferent and afferent neuromuscular coordination between the UVJ and the periureteric bladder wall is suggested by neurophysiologic studies that induce elevation or decrease in intraluminal UVJ pressure during bladder filling (Shafik, 1996). The most common anatomic obstruction of the bladder in the pediatric population is posterior urethral valves (PUVs). Reflux is present in 48% to 70% of PUV patients (Reuter and Lebowitz, 1985; Puri and Kumar, 1996; Hassan et al, 2003; Priti et al, 2004). Relief of PUV obstruction appears responsible for resolution of reflux in about one third of the patients. In one of these series, 78% of reflux resolved within 6 months of valve ablation (Priti et al, 2004). Such observations argue for the secondary nature of reflux due to elevated voiding pressures in PUV bladders. Even prostatic enlargement and its relief are associated with VUR and its resolution, respectively (Morita, 1987). In females, anatomic bladder obstruction is rare. The most common structural obstruction is from a ureterocele, which prolapses into the bladder neck (Merlini and Lelli Chiesa, 2004). In such cases, reflux in a contralateral ureter is likely due to the ensuing outlet obstruction and often resolves with decompression of the ureterocele. In general, then, if relief of the obstruction results in rapid reflux resolution, the reflux was likely secondary. In contrast to anatomic obstruction, neurofunctional etiologies of elevated bladder pressures also predispose to VUR. In particular, neurogenic bladder associated with spina bifida is at risk for reflux (Bauer et al, 1982). This fact must be borne in mind during evaluation of the child with UTI. Special attention to the potential for occult spinal dysraphism including sacral dimple or hairy patch, gluteal cleft abnormality, diminished rectal tone, or significant constipation or encopresis should prompt consideration of investigation for coexistent spinal cord abnormalities. Urodynamic extremes that predispose to reflux in the absence of overt neurologic pathology may also exist. Some studies suggest that a secondary aspect to neonatal reflux is a peculiarity of male infants. In a study by Yeung and colleagues (1998), 22 of 24 refluxing infants showing urodynamic evidence of instability (overactivity), inadequate, or obstructive voiding patterns were male. Normal or immature voiding patterns were observed in all infants in the nonrefluxing control group, of which 16 of 21 were male. In infants, higher voiding pressures are associated with reflux, particularly in boys (Chandra et al, 1996), and may contribute to the male preponderance of reflux in infants. Urodynamic evaluation suggests these elevated infant bladder pressures may be due to inadequate sphincter relaxation (Chandra and Maddix, 2000) during this stage of development. However, detrusor activity in such infants is largely normal during filling, with slightly diminished bladder capacities in some (Podesta et al, 2004), though uninhibited activity during filling has been observed, again predominantly in male infants (Yeung et al, 1998). Considering that the high prevalence for reflux in infants coexists with urodynamic evidence of elevated voiding pressures, these observations suggest that infant voiding patterns may be a part of normal development. Thus even though the UVJ matures with age, infant voiding patterns predispose to a form of secondary reflux that resolves with normalization of urodynamic parameters as these infants grow older. In older children, acquired abnormalities in voiding parameters commonly known as dysfunctional voiding or dysfunctional elimination have been associated with reflux. The precise cause of voiding dysfunction is variable but may evolve from a persistence of the expected early attempts to suppress bladder contractions during the toilet training months by volitional contraction of the external sphincter (Allen, 1985). If this behavior becomes prolonged or intensifies, often driven by the child’s overwhelming desire for continence, bladder voiding pressures increase. Continence is gradually exchanged for incomplete emptying, resulting in a higher UTI risk. Although investigation of UTI might necessarily diagnose some subjects with persistence of primary reflux, the elevated bladder pressures gradually distort bladder and UVJ architecture and may create (secondary) reflux (Koff and Campbell, 1992). Structural failure of the UVJ is likely a critical determinant because high voiding pressures of approximately 100 cm of water are common in normal bladders and structurally intact nonrefluxing UVJ. Indeed, with structural failure of the UVJ, reflux occurs easily and at low voiding pressures or during early filling and is a poor prognostic factor for reflux resolution (Koff and Campbell, 1992; Hinman et al, 2002). Uninhibited bladder contraction is the most common urodynamic abnormality associated with reflux in neurologically normal children. In one study of 37 girls with “primary” reflux, 75% had uninhibited contractions (Taylor, 1982). However, the observation that treatment of such patients with oxybutynin can virtually eliminate reflux in up to 80% of refluxing ureters strongly argues that an overactive bladder can frequently be responsible for reflux, either by causing secondary reflux or perpetuating primary reflux (Koff and Murtagh, 1983; Homsy et al, 1985; Seruca, 1989). Thus it is apparent that primary and secondary reflux may not always be mutually exclusive or that what is perceived as primary reflux in some children may, in fact, be secondary to abnormal voiding patterns. The previous discussion clearly suggests that multiple opportunities exist for modifying the course of reflux if secondary causes are appreciated, identified, and treated. Van Gool and coworkers (1992) identified that 18% of children enrolled in the European arm of the International Reflux Study in Children harbored voiding dysfunction associated with more frequent UTI and greater persistence of reflux compared with those subjects without voiding dysfunction. Similar findings were reported from the recent Swedish Reflux Trial in which a cohort of children 1 year of age to younger than 2 years of age with grade 3 and 4 reflux were assessed and followed for 2 years for lower urinary tract dysfunction prevalence and type (Sillen et al, 2010). Twenty percent of children harbored some form of dysfunction at entry. This dysfunction prevalence increased to 34% at 2 years of follow-up. A negative correlation was found between dysfunction at follow-up and reflux improvement (P = .002). In addition, scintigraphic renal abnormalities (defined in Brandström et al, 2010a) at study entry and at follow-up were also associated with dysfunction (P = .001). Failure to address voiding abnormalities can also adversely affect outcome of antireflux surgery (Koff et al, 1998). Indeed, compelling meta-analytic evidence recently compiled by the American Urological Association (AUA) Panel on VUR Guidelines now suggests that bladder and bowel dysfunction (BBD) is by far one of the most critical and modifiable variables that affect VUR management and attendant UTIs. Composite study analysis now indicates that BBD is associated with a higher incidence of UTIs while on antibiotic prophylaxis, as well as after surgical correction of VUR, with less VUR resolution at 24 months from diagnosis and with reduced success of endoscopic surgery. In the studies selected by the Panel that qualified as having acceptable levels of evidence, BBD did not appear to reduce the success of open surgical reflux correction (Fig. 122–2). Figure 122–2 Dysfunctional elimination syndrome (DES) and urinary tract infection (UTI). CAP, continuous antibiotic prophylaxis. (From Peters CA, Skoog SJ, Arant BS, et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol 2010;184:1134–44.) Thus although dysfunctional elimination syndromes are discussed in detail elsewhere in the text, a thorough evaluation of the toilet-trained child with reflux must recognize dribbling, urgency, or incontinence as signs of coexisting voiding disorders. Girls will also exhibit procrastination about voiding or demonstrate curtsying behavior, and boys may squeeze the penis, in attempts to suppress bladder contractions. The close proximity of the bladder and anal outlets often leads to sympathetic contraction of the anal sphincter as well, resulting in the frequent association of constipation and encopresis with reflux and UTI, in what is probably a mutually aggravating pattern (O’Regan and Yazbeck, 1985; O’Regan et al, 1986; Chase et al, 2004). Constipation must be recognized and eliminated as much as possible in order to establish optimal conditions for successful spontaneous or surgical resolution of reflux. The initial report of McGuire and colleagues (1981) suggested that pressures in excess of 40 cm of water measured at full capacity is associated with reflux and upper tract deterioration. Treatments to maintain pressures below this value result in significant reflux resolution (Flood et al, 1994). Reflux is not a general cause of UTI. In the absence of bladder symptoms or inflammation, reflux is most readily considered a clinical accelerant of bacteriuria, by mechanically delivering infected urine to the renal pelvis. Infection-related cystitis is expected to incite bladder irritability and dysuria, upsetting the voiding pattern and lowering the threshold for reflux in a given UV junction. However, animal studies differ on whether infection itself can perpetuate UV reflux. In primate studies, surgically created reflux followed by introduction of pathogenic bacteria into the bladder was associated with reflux persistence (Roberts et al, 1988), compared with spontaneously occurring primary reflux in the presence of chronic infection (Lewis and Roberts, 1986), where infection did not delay reflux resolution. Significant hydroureter and hydronephrosis associated with high-grade reflux could, in theory, act as a reservoir for the repeat antegrade reintroduction of pathogenic organisms to the bladder. Colonized urine might then cyclically reflux retrograde to the upper tracts. Similarly, ureteral atony secondary to the effects of endotoxin could fail to expel infected urine from the upper tracts, but this does not appear to reduce the ultimate resolution of reflux (Roberts and Riopelle, 1978). Indeed, the dilated upper tracts seen in high-grade reflux explored in the Swedish Reflux randomized prospective controlled trial were associated with a higher rate of recurrent febrile UTI in patients on surveillance without antibiotic prophylaxis (57%) (Brandström et al, 2010b). Reflux correction or antibiotic prophylaxis reduced the recurrent infection rate to approximately 20% (P = 0.0001). Interestingly, the finding was restricted to girls, which may reflect an anatomic predisposition to bacterial colonization of the bladder in girls versus boys; the febrile nature of the infection was likely due to reflux washing of bacteria to the upper tracts and renal parenchyma. Grading systems generally exist to help prognosticate the behavior of the disease they classify. In 1981 the International Reflux Study Committee proposed a system of five grades of reflux that remains in current use today in North America (Duckett and Bellinger, 1982; Lebowitz et al, 1985). Five grades of reflux are currently used to depict the appearance of the ureter, renal pelvis, and calyces as seen on the radiographic contrast images generated by the voiding cystourethrogram (Table 122–3 and Fig. 122–3). Using such a system serves several purposes. Grading standardizes the description of the degree of reflux for clinical management of the individual patient, as well as for grouping research subjects in the design of clinical studies and trials. Grading facilitates documenting the natural history of the reflux process in the individual patient. It also permits establishing quantitative associations between reflux and other clinical parameters, in order to determine whether such associations hold multivariate clinical relevance. Most importantly, description of initial grade in primary reflux is the most significant parameter associated with prediction of reflux resolution (see later). Table 122–3 International Classification of Vesicoureteral Reflux Despite the widespread use of the five-point grading system, several shortcomings exist. For example, the expected concordance between ureteral and calyceal dilation does not always occur (Fig. 122–4). Either the ureter or the calyces may demonstrate dilation out of proportion to the calyces or ureter, respectively. Whether this reflects an anomaly in the biomechanical tissue properties or peristaltic activity of the excessively dilated structure, as compared with the typical refluxing upper tract is unknown. Nevertheless, such anatomy is difficult to grade using the current system. Similarly, it is unknown whether the propensity to either scarring in the face of infection or reflux resolution is altered in such systems. Attempts have also been made to grade reflux using radionuclide cystography (RNC). Because RNC does not provide discrete images of the ureteral and calyceal architecture required to assign reflux grade, classifying reflux by RNC is difficult. Alternative RNC grading has been proposed (Zhang et al, 1987). This provides reasonable concordance to the objectives of the classic grading system by collapsing grades 2 and 3, as well as grades 4 and 5, into low-grade and high-grade reflux, respectively (Fig. 122–5). The impact of the reduction in grading detail from five grades (1 to 5) to two (low and high) on understanding reflux pathophysiology and on the design of clinical studies has yet to be determined. Although grading is purely a classification of the appearance of contrast in the upper tract, ascribing an absolute value to the grade has the potential to cast the reflux in unequivocal terms, to the exclusion of other factors that modulate reflux at the time the images are acquired. Inherent bladder dynamics, the bladder outlet, and even ipsilateral UVJ obstruction all influence the degree of reflux at any given assessment (Lebowitz, 1992). Grade, reflux detection, and reflux resolution are all interrelated and are influenced by the degree of bladder filling, the voiding cycle, the number of filling cycles during the contrast cystogram, and whether the reflux occurs during filling or only during voiding. None of these parameters is built into the grading system for reflux used currently. However, given the principal role of a reflux grading system is to help prognosticate reflux resolution, future incorporation of other parameters may provide additional clinical acumen. Finally, it must be remembered that reflux grade is still presently determined by an imaging study that requires invasion of a sensitive anatomic region. For a given reflux grading result, the aforementioned variables of bladder dynamics, filling, and voiding cannot necessarily be replicated with certainty in a given patient each time he or she is studied. It is reasonable to speculate that reflux grade could easily vary up or down by at least one grade if sequential studies were performed in a given patient. Thus the entire reflux literature itself, which historically reports results in terms of five separate grades, must be considered with some circumspection because the veracity of grade at the time of the contrast study may be, to some extent, arbitrary. In infants, specimen collection will often entail the placement of an adhesive urine collection bag over the genitalia. Although topical cleansing of the area is reasonable to reduce contamination and false-positive cultures, care must be taken to avoid introducing the disinfection agent into the specimen. Unfortunately, routine practice is so variable that such guidelines are often difficult to follow or confirm and microbial contamination of bagged specimens is common. Conversely, this method is most useful if the resulting urine culture is negative. Although fevers are common in infants, UTI comprises only 5% of children presenting with fever (Hoberman and Wald, 1997). Thus the ability to easily rule out a UTI in the febrile infant with reflux facilitates management. If there is a high clinical suspicion for UTI in an infant, the more accurate method is to obtain a catheterized specimen. Even this method may prove suspect in the uncircumcised infant male whose preputial skin is not yet retractable to easily reveal the urethral meatus. In this and other instances where the urethral route is difficult or compromised in patients of any age, a suprapubic needle aspiration of bladder urine provides the most accurate method of obtaining a urine sample. In the younger child, however, repeated catheterization for specimen acquisition and radiographic assessment (see later) should be considered carefully. This hitherto routine practice is coming under greater scrutiny as the long-term sequelae of this invasive maneuver become appreciated (Stashinko and Goldberger, 1998) and could become a factor in patient compliance with follow-up (and therefore management decisions for reflux). Numerous factors support a search for reflux in the patient with UTI. The probability of finding VUR in children with a UTI is 29% to 50% (Anonymous, 1981). Furthermore, in some patients, higher grades of reflux may be associated with various degrees of existing renal parenchymal maldevelopment (see embryology section earlier) (Nakai et al, 2003). In addition, because reflux tends to resolve over time, it is reasonable that UTI is more commonly associated with reflux in younger patients (Smellie et al, 1981b), whose renal parenchyma is at more risk for scarring following a given pyelonephritis, compared with the older child. Smellie also suggests that the presence of reflux does not usually provide any unique clinical features in the patient with a UTI. Thus as with the search for sibling reflux, radiologic investigation of the patient with UTI is tailored to those patients who are placed at greatest renal functional risk from the presence of VUR. For this reason, radiographic investigation for VUR has generally been directed to children younger than 5 years old, all children with a febrile UTI, and any male with a UTI regardless of age or fever, unless sexually active. The high repeat UTI rate after a first UTI has prompted a recommendation for some form of VUR work-up (Fig. 122–6). However, the current state of the VUR debate makes it difficult to know which patients, by such evaluations, might harbor clinically significant reflux, given how common UTI is in children. Some reassurance can be provided if a voiding study is negative for VUR. However, the parental concern over the invasiveness of the cystogram may first limit the evaluation to ultrasonography only, to rule out any existing gross structural defects. As such, taking the appropriate voiding, fever, and family histories into account, a sonographic study of the bladder and kidneys can be considered a reasonable minimum evaluation in the infant or child following a UTI, if a cystogram is deemed overly aggressive by the family or treating physician. Even the detection of reflux following treatment for UTI could be of questionable significance if the child is older and/or no fever accompanies the UTI. On the other hand, the presence of structural renal anomalies or significant asymmetry would support proceeding with a cystogram. More recent studies of racial predilection for reflux have supported a lower prevalence in children of African origin (Askari and Belman, 1982; Horowitz et al, 1999), but the prevalence in children of Hispanic origin (Pinto, 2004) is similar to that of Caucasians. Figure 122–6 Urinary tract infection (UTI) history. (From Peters CA, Skoog SJ, Arant BS, et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol 2010;184:1134–44.) The advent of prenatal ultrasound has likely augmented the detection of asymptomatic reflux in newborns, as a result of mass surveillance for persistent postnatal hydronephrosis and the ensuing performance of cystography in these infants. It must be remembered that the sonographic finding of hydronephrosis is generally much more common than hydronephrosis due to a renal moiety actively refluxing at the time of the sonogram. Indeed, there is little correlation between the degree of antenatal hydronephrosis and the existence of reflux (Farhat et al, 2000). A corollary study also demonstrated normal postnatal ultrasounds in 25% of patients with VUR and antenatal hydronephrosis (Lebowitz, 1993). The basis of reflux detection lies in demonstrating the retrograde passage of an imaging contrast material from the bladder to the ureter and renal pelvicalyceal system. The currently available methodologies require a source of contrast agent in the bladder. Two approaches, the so-called indirect and direct cystograms, can be performed depending on whether the contrast enters the bladder indirectly following excretory urography or directly, usually by urethral catheterization. Indirect cystography, although avoiding the invasive nature of urethral catheterization, is prone to false-positive interpretation due to contrast that does not originate from the bladder, remaining in the ureter or pelvis after filtration and antegrade passage, and false-negative results in lower grades of reflux (Conway et al, 1975). However, it has been suggested that the value of indirect cystography may lie in ruling out the presence of VUR (Carlsen et al, 1986). The voiding cystourethrogram (VCUG) and radionuclide cystogram (RNC) therefore are the two common forms of direct cystography and constitute the present-day gold standard approaches to reflux detection. More recently, to eliminate the need for ionizing radiation, some studies have demonstrated a growing interest in sonographic detection of reflux using either color Doppler imaging (Haberlik, 1997; Oak et al, 1999; Galia et al, 2004) or echo-enhancing contrast agents (Berrocal et al, 2001; Darge et al, 2001; Darge and Troeger, 2002; McEwing et al, 2002; Tasic and Todorovska, 2003; Valentini et al, 2004; Vassiou et al, 2004; Darge et al, 2005). Direct imaging of reflux is affected by several parameters. These include bladder contraction during voiding, the fluid volume instilled into the bladder, and presence of infection and therefore inflammation of the UVJ mucosa. Reflux may occur during filling or only during the active bladder contraction associated with voiding. Consequently, if a patient is unable to void in the artificial setting of the radiography suite, false-negative results may ensue. More importantly, even during voiding, reflux may not be demonstrated on a single filling-voiding cycle. Several studies have demonstrated a roughly 12% to 20% greater detection rate for VUR if a cyclic study is performed (Paltiel et al, 1992; Papadopoulou et al, 2002; Novljan et al, 2003). A cyclic VCUG involves a second or third cycle of bladder filling and emptying under fluoroscopic observation. A similar cyclic strategy is commonly employed for the RNC as well (Fettich and Kenda, 1992). Reflux may also be demonstrated during catheter filling of the bladder. Because filling assumes far lower intravesicle pressure than that of voiding, reflux during filling, or passive reflux, is generally considered a poor prognostic sign for reflux resolution and suggests the presence of a fixed decompensation of the UVJ. This is a common finding in patients with acquired or neurogenic voiding dysfunction, wherein high-resistance voiding gradually remodels the bladder wall and UVJ, leading to complete failure of the latter’s antireflux mechanism, and immediate reflux at virtually any volume of the filling phase (Koff, 1992). This is in contrast to reflux that only occurs during the higher-pressure milieu of bladder emptying and contraction. Thus technical inconsistencies in the ratio of instilled volume-to-bladder capacity during the radiographic technique can lead to variations in rates of reflux detection: if the bladder is overfilled or underfilled for a given degree of progressive UVJ incompetency, reflux may be overdetected or underdetected, respectively. One difficult dilemma in the performance of voiding studies involves cystography during active infection. On one hand, some UVJs maintain only borderline antireflux mechanisms, which are competent in a sterile milieu but become incompetent from edema and inflammation associated with mucosal inflammation during cystitis. Such patients may have VCUG studies negative for reflux in the absence of infection but suffer from repeated pyelonephritic episodes. Cystograms in such patients may demonstrate reflux if obtained during active infection. On the other hand, evoking reflux during an active cystitis, by definition, will transmit bacteria to the upper urinary tract and renal pelvis and risks iatrogenic pyelonephritis. Nevertheless, the general consensus has been to delay the voiding study for at least a week or longer to allow for adequate recovery from the acute infection episode (Craig et al, 1997). Only if it is imperative to make the diagnosis of reflux in children with a history of recurrent pyelonephritis and repeatedly negative voiding studies in the intercurrent periods should cystography during UTI be considered. The VCUG is a fluoroscopic study that provides information on both the functional dynamics and structural anatomy of the urinary tract. The detailed technique of the VCUG is discussed more fully elsewhere in the text. Bladder contrast is instilled by gravity following urethral catheterization. Bladder capacity is recorded when contrast influx ceases. Static images record bladder contour, presence of diverticula or ureteroceles, grade of reflux, configuration and blunting of calyces, and intrarenal reflux. Passive or active reflux is demonstrated dynamically during fluoroscopy while filling and voiding, respectively. In addition, bladder neck anatomy, funneling or dilation, and urethral patency are parameters derived from the VCUG. Delayed or postvoid films are crucial in documenting clearance of contrast from the upper tracts because retained contrast, particularly with dilated pelvicalyceal systems, could signify the presence of a concomitant UPJ obstruction (UPJO), either primarily or secondarily due to distortion of the UPJ by massive retrograde filling of the pelvis by the reflux (Hollowell et al, 1989). If both the UPJ and UVJ meet criteria for operative repair, the UPJ should be repaired first to avoid the incipient obstruction that may ensue if resistance is added to the UVJ when reflux is corrected (Hollowell et al, 1989). With care, both processes may be repaired simultaneously when it is clear that they are independent significant problems. The RNC has historically enjoyed a reputation for requiring approximately 1% the radiation exposure generated by the VCUG (Blaufox et al, 1971; Diamond et al, 1996a). Presently, the reduced radiation requirements of modern digital techniques have significantly narrowed the difference between fluoroscopy and RNC. Although little anatomic detail is afforded by the RNC, it is ideal as both a screening modality and for monitoring the natural history or surgical follow-up of reflux. In contrast to the VCUG, the instilled bladder contrast material, usually Technetium Tc99 pertechnetate, is itself the radiation source. Reflux is detected on scintigraphic gamma camera images (Fig. 122–7). Lack of confounding imaging densities typical of fluoroscopy, as well as the ability to obtain prolonged exposures, allow for greater sensitivity of the RNC in grade 2 to 5 reflux. Ironically, grade 1 reflux into the distal ureter is often poorly detected due to the overlying exposure generated by contrast within the bladder itself. Thus RNC and VCUG imaging can be used complementarily, balancing radiation exposure with the need for dynamic information and anatomic detail. Notwithstanding the forgoing discussion, modern digital fluoroscopic equipment has further reduced the radiation exposure of conventional fluoroscopy, thereby narrowing the difference in radiation exposure between the two modalities. Another modality gaining popularity in some centers is ultrasonic cystography to detect reflux. Modern transducers coupled with the use of echo-enhancing contrast agents can image reflux well in older children (Novljan et al, 2003; Riccabona et al, 2003; Tasic and Todorovska, 2003; Galia et al, 2004), but the technique remains of limited use in neonates (McEwing et al, 2002). Furthermore, while radiation exposure is eliminated, bladder catheterization remains a necessary feature with this approach. The well-recognized distress of the family and patient associated with urethral catheterization, particularly in children, coupled with the advent of low-morbidity outpatient correction of reflux using endoscopic approaches appears to be challenging conventional reflux management and therapy. All aspects of VUR including modern incidence of renal scarring and failure, peak ages of renal susceptibility, and indications for reflux correction, including the assumption that permanent correction of reflux is an absolute necessity, are coming under scrutiny. This has given rise to added pressure to avoid bladder catheterization. The cystogram can have traumatic aftereffects in young patients (Stashinko and Goldberger, 1998; Elder, 2005) and must be approached with sensitivity and awareness of the developmental stage of the patient. Sedation (Stokland et al, 2003), topical urethral anesthetic (Gerard et al, 2003), and more recently hypnosis (Butler et al, 2005) have all been effective in mitigating the deleterious psychologic effects of cystography. Nevertheless, it is clear that parental perception of the nature of the medical modalities involved in reflux management will influence their choice of therapy for their children (Ogan et al, 2001). This should in no way be construed as license for the abandonment of conventional observational therapy including antibiotic prophylaxis and periodic cystography in favor of immediate reflux correction in all patients (Aaronson, 2005). Rather, the evolution of less invasive imaging modalities for reflux detection and reduced morbidity of reflux correction should be encouraged in parallel. Hansson and colleagues (2004) retrospectively reviewed 303 children younger than 2 years of age who had undergone evaluation by DMSA and VCUG within 3 months of their first UTI. Although 82% of these infections were febrile, VUR was found in only 26% of the children (80/303), 66% of whom had abnormal DMSA scans, while no abnormalities were detected in the remaining 27 patients. An approach based on identifying renal cortical abnormalities before obtaining a VCUG would have identified the 66% of children with VUR presumably at risk for further scarring, and it would have excluded 120 children (40%) without VUR or renal abnormalities from an unnecessary VCUG. In a follow-up study from the same institution, Preda and colleagues (2007) prospectively confirmed these observations in 290 children younger than 1 year of age with UTI (79% febrile) with VCUG and DMSA renal scans. Fifty-one percent of the patients had positive scans including 85% (44/52) of the children later found to have VUR. Seven of the eight cases of VUR with normal DMSA scans were low grade, the remaining boy having grade 3 VUR with no acquired renal scars in follow-up despite an episode of breakthrough UTI. Modern management of reflux does not include routine cystoscopy. It is rare for cystoscopy to add any information that will alter management of a patient with reflux, either at the time of initial diagnosis or during follow-up. Its routine use, especially in children with UTI or reflux, should be considered an anachronism. Similarly, the appearance and configuration of the UOs and intramural tunnel length that are afforded by cystoscopy and once considered useful parameters have over time provided little correlation with either the diagnosis or grade of reflux (Duckett, 1983). Cystoscopy can provide useful information immediately before open surgery, such as confirmation of orifice position and duplication and the proximity of diverticula to the orifice, and clarify urethral patency if indicated. Similarly, such cystoscopic parameters will become immediately available in all patients at the time of endoscopic reflux correction. A recently developed, although still controversial (Elder, 2005), cystoscopic modality termed the PIC technique (Positioning of the Instillation of Contrast at the UO) purports to detect reflux under general anesthesia in patients with a history of febrile UTIs but a normal VCUG. The technique of PIC cystography (Edmondson et al, 2006) involves cystoscopy using a 9.5-Fr or 14-Fr rigid cystoscope. With the bladder empty, the cystoscope beak is positioned close to and facing the UO. Contrast is instilled at the UO using the irrigation port of the cystoscope from a height of 1 meter above the bladder. Fluoroscopic spot imaging is done simultaneously with the instillation. PIC-VUR is confirmed if retrograde flow of contrast into the ureter/kidney pelvis is observed. The bladder is emptied before the procedure is repeated on the contralateral side. However, a meta-analysis of 63 studies assessing the value of routine diagnostic imaging after initial UTI in children for the prevention of renal damage found no accurate evidence to support this practice (Dick and Feldman, 1996). Many observational studies raised concern for the sequelae of UTIs and highlighted the potential for successful intervention. The reason to image the upper tracts following a UTI may occur with or without knowledge of the reflux status of the lower tract. Reflux status may be known, suspected, or completely unknown. These three considerations, coupled with the age of the patient, gender, race, family history of reflux, and bladder functional status serve as a guide to selective imaging, which attempts to balance intensity of imaging studies with propensity for renal damage. Ultrasound lends itself well to quantitative assessment of renal dimensions (Rodriguez et al, 2001; Chen et al, 2002), which can then be used to follow renal growth over time. Renal growth can be referenced to standard renal growth curves. In reflux diagnosed in the neonatal period, baseline renal dimensions are obtained and appropriate renal growth can be monitored over time. The impact of any intercurrent febrile urinary infection can be gauged by observing the effects on renal growth. Similarly, if the UTI history between follow-up visits for reflux is unclear, serial assessment of renal dimensions can help the urologist advise on the need for further assessment of renal function by scintigraphy or on the need for reflux correction. Indeed, in the presence of reflux, modern postnatal renal sonography provides excellent correlation between renal length and scintigraphic hypoplasia (Farhat et al, 2002a).
Historical Perspective
Demographics
Prevalence
Gender
Reflux in the Fetus
Age
AGE (yr)
INCIDENCE (%)
<1
70
4
25
12
15
Adults
5.2
Race
Inheritance and Genetics
Sibling Reflux
Genes Involved
Embryology of the Ureterovesical Junction
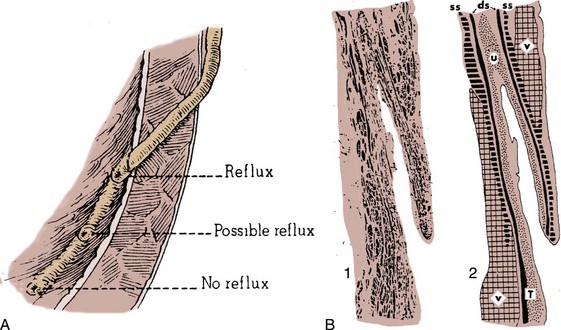
Functional Anatomy of the Antireflux Mechanism
Etiology of Vesicoureteral Reflux
Secondary Reflux
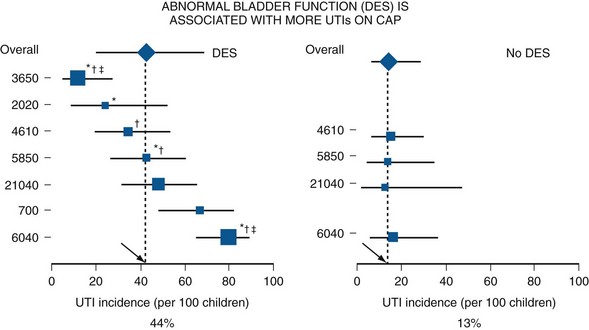
Clinical Correlates
Lower Tract Urinary Tract Infection and Reflux
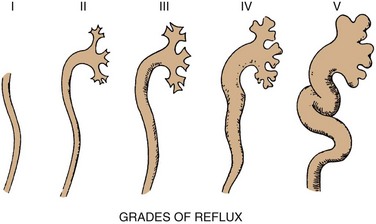
Grading of Reflux
GRADE
DESCRIPTION
1
Into a nondilated ureter
2
Into the pelvis and calyces without dilatation
3
Mild to moderate dilatation of the ureter, renal pelvis, and calyces with minimal blunting of the fornices
4
Moderate ureteral tortuosity and dilatation of the pelvis and calyces
5
Gross dilatation of the ureter, pelvis, and calyces; loss of papillary impressions; and ureteral tortuosity
Diagnosis and Evaluation of Vesicoureteral Reflux
Confirmation of Urinary Tract Infection
Evaluating Urinary Tract Infection
Assessment of the Lower Urinary Tract
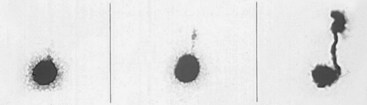
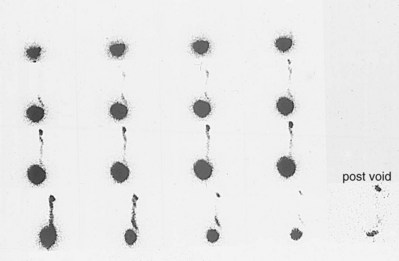
Cystographic Imaging
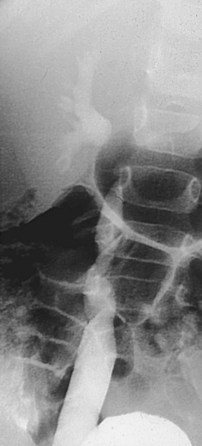
Diagnostic Controversies: Challenging the Assessment of Reflux
Top-Down Approach
Cystoscopy and the Positioning of the Installation of Contrast Cystogram
Assessment of the Upper Tract
Rationale for Serial Assessment of Upper Tracts
Renal Sonography
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vesicoureteral Reflux