Jack Christian Winters, MD, Joanna Maya Togami, MD, Christopher J. Chermansky, MD The bones of the pelvis provide the scaffold on which the soft tissue supports (muscles, ligaments, and fascia) are anchored. They include paired innominate or hip bones on either side of the sacrum, which comprise the pelvic girdle. The innominate bones are further divided into the ileum, the ischium, and the pubis. The ischial spine provides attachment for the arcus tendineus fasciae pelvis (ATFP), the sacrospinous ligament, and the coccygeus muscle (Fig. 72–1). The obturator foramen is formed by the superior pubic ramus above, the pubic body and inferior ramus medially, and the anterior border of the ischial body below (Newell, 2005) (Fig. 72–2). Figure 72–1 Left hemipelvis with ligaments. (From Newell R. Pelvic girdle, gluteal region and hip joint. In: Standring S, editor. Gray’s anatomy: the anatomical basis of clinical practice. 39th ed. New York: Elsevier; 2005.) Figure 72–2 Left obturator foramen and membrane. (From Newell R. Pelvic girdle, gluteal region and hip joint. In: Standring S, editor. Gray’s anatomy: the anatomical basis of clinical practice. 39th ed. New York: Elsevier; 2005.) The pelvic floor comprises all of the soft tissues that essentially hold the pelvic viscera in place at the base of the bony pelvis and the levator ani muscle complex. The pelvic floor is a three-dimensional structure that functions as a unit (Hurt, 2000). The pelvic diaphragm is composed of the striated pubococcygeus, iliococcygeus, and coccygeus (Fig. 72–3). The levator ani muscles are composed of the pubococcygeus and iliococcygeus. The coccygeus is also termed the ischiococcygeus and is attached to the lateral margins of the coccyx and fifth sacral segment (Mundy, 2005). It is attached laterally to the ischial spine. The iliococcygeus is attached to the ischial spine, the arcus tendineus levator ani laterally and posteriorly, the tip of the sacrum, and the coccyx. The pubococcygeus is attached to the back of the pubis, and it courses lateral to the urethra (in males it is the pubourethralis, and in females, because it forms a sling around the vagina, it is the pubovaginalis). In both men and women, fibers of the pubococcygeus attach to the perineal body (Mundy, 2005). The pubococcygeus compresses the visceral canals, which cross the pelvic floor. The puborectalis portion of the pubococcygeus helps to create the anorectal angle. Contraction of the puborectalis causes the rectoanal junction to move toward the pubic symphysis, which is critical in maintaining fecal continence (Rogers, 2003). Although the muscles are referred to separately, like other structures of the pelvic floor the boundaries are often difficult to delineate and they perform similar physiologic functions (Mundy, 2005). The posterior levator ani group (iliococcygeus and coccygeus) fuses in the midline and attaches to the coccyx. The complex formed by this fusion is the levator plate, which serves as a supporting structure for the upper vagina and cervix, This serves not only to stabilize the upper vagina in a horizontal plane but also to provide a protective mechanism preventing downward forces onto the perineal body (Wall and Menafee, 2002). Figure 72–3 Muscles of the female pelvic diaphragm. (From Anderson J, Genadry R. Anatomy and embryology. In: Novak’s text of gynecology. 13th ed. Philadelphia: Lippincott Williams & Wilkins; 2002, with permission.) Beneath the pelvic diaphragm is the diamond-shaped urogenital diaphragm (Fig. 72–4). The boundaries are the pubic symphysis anteriorly, the tip of the coccyx posteriorly and laterally, and the ischiopubic rami on either side. It can be further divided anteriorly and posteriorly by imagining a line between each ischial tuberosity. This results in the urogenital triangle anteriorly and the anorectal triangle posteriorly. The deep transversus perinei muscle, also referred to as the perineal membrane, is contained by the urogenital triangle, in which the urethra and vagina traverse. In contrast to the perineal membrane in males, which is a sheetlike structure, the perineal membrane in females is a three-dimensional structure that has two regions, dorsal and ventral (DeLancey, 1999; Stein and DeLancey, 2008) (Fig. 72–5). The dorsal region is attached to the perineal body and the lateral wall of the vagina via the ischiopubic rami. The ventral region is part of a solid three-dimensional mass that is contiguous with the paraurethral and paravaginal connective tissues. This portion contains the compressor urethrae and urethrovaginal sphincter muscles of the distal urethra (Stein and DeLancey, 2008). (From Mundy A. True pelvis, pelvic floor and perineum. In: Standrinak W, editor. Gray’s anatomy: the anatomical basis of clinical practice. 39th ed. New York: Elsevier; 2005.) (From DeLancey JO. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol 1999;180:815–23.) The anorectal triangle contains the external anal sphincter, which attaches to the anococcygeal ligament and fuses to the superficial transverse perinei muscle. External to the urogenital triangle are the external genital muscles, bulbospongiosus, ischiospongiosus, and superficial transversus perinei. The hymen is located just inside the labia minora and is the fixed point of reference recommended by the International Continence Society Committee on Standardisation of Terminology, Subcommittee on Pelvic Organ Prolapse and Pelvic Floor Dysfunction to grade prolapse (Bump et al, 1996). This is in contrast to the term introitus, which is ill defined. The urogenital hiatus is the opening within the levator ani muscle through which the urethra and vagina pass. Because the rectum is attached directly to the muscles at this level it is not within the urogenital hiatus. The hiatus is supported anteriorly by the pubic bones and levator ani muscles and posteriorly by the perineal body and external anal sphincter (Ashton-Miller and DeLancey, 2007). The urogenital hiatus elongates and descends with POP. The endopelvic fascia is a network of fibromuscular tissue located between the peritoneum and the levator muscles. It surrounds and attaches the bladder, uterus, vagina, and rectum to the pelvic walls, thereby stabilizing the pelvic viscera. The endopelvic fascia is one continuous unit; however, distinct areas are named separately. The parametrium (broad, cardinal, and uterosacral ligaments) attaches the uterus and upper vagina to the pelvic sidewall. The paracolpium (the arcus tendineum levator ani and the ATFP) attaches the vagina to the pelvic sidewalls. In his landmark article, DeLancey (1992) described the supports of the vagina and conceptually divided them into three parts according to the region of vaginal support (Fig. 72–6). The structures supporting the uterus and the cephalad 2 to 3 cm of the vagina comprise level I support, and these fibers originate from the greater sciatic foramen, the sacroiliac region, and the lateral sacrum. The fibers are primarily vertical in their orientation and are the longest fibers of the endopelvic fascia, thereby suspending the uterus and upper vagina, and comprise the cardinal/uterosacral ligament complex. Level II support is at the mid vagina. These fibers are shorter than level I support but longer than those at level III. The orientation of the attaching fibers is lateral, and they are more dense than the cardinal/uterosacral ligament complex. The endopelvic fascia splits at this level to encompass the bladder and urethra such that the abdominal leaf is still named the endopelvic fascia and the vaginal leaf is the pubocervical and periurethral fascia. Posteriorly, the endopelvic fascia, which attaches laterally to the superior fascia of the levator ani muscles, is the rectovaginal fascia. Level III support of the vagina starts at the introitus and extends 2 to 3 cm above the hymenal ring. In this most distal location there is no intervening paracolpium and the vagina is fused directly to the urethra and is embedded in the connective tissue of the perineal membrane (urogenital diaphragm). Laterally it blends into the medial margins of the levator ani muscles, and posteriorly it blends into the perineal body. (From DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 1992;166:1717–28.) The endopelvic fascia is a composite of tissues of fibers embedded in a matrix. The variability of this tissue matrix may lead to its inherent weakness and inconsistency in reconstructive surgery. In fact, fresh strips of pubocervical fascia have been shown to shorten 15% to 20% of its length (Richardson, and DeLancey, 2000). Thus, these tissues are supportive and contractile. Histologically, this tissue is unlike abdominal wall fascia or the fascial coverings of other muscles such that they lack the organization of the fascial coverings of the skeletal muscle. Because the urethra is fused to the anterior vaginal wall for much of its length, the supporting structures of the urethra and distal anterior vagina are one in the same. The major components are the endopelvic fascia (periurethral and pubocervical fascia), ATFP, and the levator ani muscles (Ashton-Miller and DeLancey, 2007). At this level the endopelvic fascia surrounds the vagina and attaches to the ATFP bilaterally. Each ATFP is attached to the pubic bone anteriorly and to the ischial spine dorsally. This structure appears as a band closer to the pubic bone, and as it courses toward the ischial spine it fans out into a broad aponeurotic structure. Laterally it merges with the levator ani muscles at the confluence of the iliococcygeus and obturator internus. This support is provided by the cardinal/uterosacral ligament complex, which are level I supports as described by DeLancey (1992). They originate from fibers of the pelvic sidewall and extend to the area around the greater sciatic foramen and extend to the second, third, and fourth sacral segments. From there they fan out as they attach to the cervix and the upper vagina. The medial edge of this complex is the area of the uterosacral ligaments. The uterosacral ligaments are visible and palpable with traction of the cervix or the cuff of the vagina after hysterectomy. The fibers encircle the cervix and in aggregate are called the pericervical ring. The rectovaginal fascia is also sometimes referred to as Denonvilliers fascia in the female (Richardson and DeLancey, 2000). It has more dense strands of elastin and less smooth muscle compared with the pubocervical fascia. It is stabilized in the upper vagina by the cardinal/uterosacral ligament complex, because it is contiguous with this structure. The uterosacral ligament inserts into the rectovaginal fascia just below the attachment to the posterior cervix. Because the rectovaginal fascia is connected to the sacrum and the perineal body it provides continuous posterior support. The perineal body is a condensation of fibromuscular tissue and collagen that is located in the midline between the vagina and the anus. It serves as a point of fixation for the rectovaginal fascia, the levator ani muscles, the transverse perinei muscles, and the external anal sphincter (Rosenblum et al, 2005). The levator ani muscles comprise both type I and type II fibers. Type I muscle fibers are slow twitch and provide sustained tonicity of the pelvic floor. This function serves a crucial role in providing dynamic pelvic floor support, thus taking the mechanical stress off of the endopelvic connective tissue attachments. Type II fibers consists of fast-twitch fibers that are mainly responsible for the reflex contractions of the pelvic floor associated with sudden increases in intra-abdominal pressure. Thus, the levator ani muscle complex fulfills multiple functions. First, the tonic contraction of the pubococcygeus muscle narrows the genital hiatus. Second, contraction of the pelvic floor leads to the elongation and elevation of the pelvic organs facilitating urinary and fecal continence. Additionally, posterior levator ani tonicity elevates the upper vagina and stabilizes it into a horizontal plane near the hollow of the sacrum (Fig. 72–7) (Wall and Menafee, 2002). Direct muscular damage, neuromuscular dysfunction, and inherent tissue defects may predispose to a dysfunction of the levator ani musculature. As a result, the burden of support shifts mostly to the endopelvic connective tissues. As these structures weaken, various “breaks” occur that lead to vaginal support defects. (From Wall LL, Menefee S. Incontinence, prolapse and disorders of the pelvic floor. In: Berek JS, editor. Novak’s textbook of gynecology. 13th ed. Philadelphia: Lippincott Williams & Wilkins; 2002, with permission.) Within the anterior compartment two types of defects can lead to cystoceles. The central cystocele results from attenuation or separation of the pubocervical fascia, resulting in a protrusion of anterior compartment structures through this defect. This protrusion results in the classic appearance of a loss of the rugae or vaginal folds of the anterior vaginal wall. Detachment of the pubocervical fascia and the pubourethral ligaments to the ATFP results in a lateral cystocele and can also involve the urethra. This usually results in a preservation of the rugal folds of the vaginal wall and a “rotational” prolapse of the anterior wall (Fig. 72–8). Urethroceles, which are distal anterior compartment defects, usually result in urethral hypermobility (Weber and Walter, 1997). Figure 72–8 A and B, Cystoceles. (From DeLancey JO. Relation of prolapse syndromes to symptoms. In: Kursh E, McGuire E, editors. Female urology. Philadelphia: JB Lippincott; 1994.) Apical compartment defects involve a disruption in the uterosacral/cardinal ligament complex. This defect may lead to prolapse of the uterus, the vaginal cuff after hysterectomy, and the peritoneum cul-de-sac with or without bowel (enterocele). Enteroceles are most commonly associated with apical or high posterior compartment defects; however, enteroceles may rarely occur anteriorly. Enteroceles can occur with or without vaginal vault prolapse. Complete vaginal vault prolapse contains enteroceles in 75% of patients. Waters (1956) described four types of enteroceles by etiology: congenital, pulsion, traction, and iatrogenic. Congenital enteroceles occur either from the failure of the peritoneum to fuse with the perineal body or from reopening of previously fused structures. Pulsion defects occur with increased intra-abdominal pressures, and traction enteroceles occur by a pulling of the vaginal epithelium from other prolapsing organs. Iatrogenic enteroceles are created when a surgical procedure is performed that alters the normal vaginal axis or when the pubocervical fascia and the rectovaginal septum are not reapproximated after hysterectomy (Wiskind et al, 1992). Uterine prolapse occurs with loss of support of the cardinal and uterosacral ligaments. The broad ligaments also provide uterine support and are located above insertion of the cardinal uterosacral ligaments. They are located within the leaves of the anterior and posterior peritoneum. Within this fused structure are the fallopian tubes and the round and ovarian ligaments along with their blood supply (Rosenblum et al, 2005). It is difficult to differentiate other prolapsing organs with loss of apical support high in the vagina. As such, careful dissection is often needed to identify other prolapsing organs with uterine prolapse. This reinforces the concept that the endopelvic fascia is best considered as a contiguous unit that can fail together. Consequently, the bladder, small bowel, and rectum are often found prolapsing with the uterus. Vaginal vault prolapse can occur after hysterectomy if support of the vault is not reconstituted by suspending it to the cardinal/uterosacral ligament complex. Incidence of vaginal vault prolapse after hysterectomy has been reported to be as high as 18.2%, and it can contribute to prolapse in other compartments (Richter, 1982). Because of the redundancy of the support by the endopelvic fascia, failure to reattach the suspensory component does not lead to immediate vaginal eversion after hysterectomy (DeLancey, 1992). Complex vaginal eversion is vaginal eversion associated with cystocele, rectocele, or both (DeLancey, 1992). Complex vaginal eversion has been reported as high as 67% of vault prolapse (Morley and DeLancey, 1988). In this group of patients, 7% had apical prolapse and cystocele, 30% had apical prolapse with rectocele, and 30% had prolapse with both cystocele and rectocele. The posterior vaginal compartment comprises the peritoneum of the cul-de-sac, the rectum, and the perineum. Defects in the rectovaginal fascia either in the form of attenuated fascia or site-specific tears will result in herniation of the rectum and sometimes the small bowel into the vagina. Rectoceles may be divided into low, midvaginal, or high depending on the location of loss of support, and it may present as a combination of defects. Richardson was the first to describe site-specific defects of the rectovaginal fascia in 1993 (Fig. 72–9). Defects in the cardinal/uterosacral ligament complex can result in high rectoceles and can involve enteroceles. Enteroceles are estimated to be present in 0.1% to 16% of women undergoing surgery for POP (Chou et al, 2000). Loss of support in the mid vagina from the lateral attachments to the arcus tendineus fascia rectovaginalis will result in a bulging in the midportion of the posterior compartment. The perineal body is suspended from the sacrum by the contiguous support of the uterosacral ligaments and rectovaginal septum. Any break in this support system will lead to a hypermobile perineal body that descends with increases in intra-abdominal pressures (Richardson, 1993). Separation of the perineal body at the level of the rectovaginal fascia results in perineal descent or a low rectocele. The rectocele will be visible just inside the hymen posteriorly (Fig. 72–10). If perineal body detachment is left untreated at the time of rectocele repair, a perineal rectocele can occur in which the rectum bulges into the perineal body, termed a perineal rectocele (Richardson, 1995). Figure 72–9 Locations where breaks in the rectovaginal septum have been observed in patients with posterior defects. (From Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clin Obstet Gynecol 1993;36:976–83.) Figure 72–10 Large rectocele with perineal body defect. (From Nichols DH. Rectocele and perineal defect. In: Nichols DH, editor. Gynecologic and obstetric surgery. St. Louis: CV Mosby; 1993. p. 363–85.) Because pelvic organ prolapse is, for the most part, a quality of life issue, consideration must be given to the grade of prolapse, patient’s symptoms, and the degree to which the patient’s quality of life is impaired (Novara and Artibani, 2005). In selecting surgical procedure(s), one must individualize management based on the unique clinical presentation of each patient, taking into account physiologic age, comorbidities, previous surgeries, and level of physical and sexual activity (Flynn and Webster, 2002). Once the decision has been made to proceed with surgery, it is imperative to recall that the best chance at restoring normal support and function is to reapproximate normal anatomy during the first surgery (Rogers, 2003). After the first procedure, the normal anatomic planes will no longer be present, which may add to the difficulty and complexity of subsequent surgeries. Recurrence rates increase with each attempt to surgically correct the defect(s) (Birch, 2005; Maher et al, 2006). In addition, the surgeon who operates in the region of the pelvic floor must understand the anatomic alterations that result from each technique and how that surgery will affect the continuity and function of the endopelvic fascia and its related viscera (Rogers, 2003). Repairs can be defined as restorative, compensatory, and obliterative (Van Rooyen and Cundiff, 2005). In patients whose comorbidities preclude prolonged surgery, obliterative procedures provide symptom relief with minimal morbidity. These procedures are contraindicated in patients who wish to remain sexually active. For those patients who have discrete defects in the fibromuscular layer of the endopelvic fascia, restorative repairs are an appropriate option. These repairs correct the defined defects in the native tissues by utilizing endogenous support structures. For patients whose native tissues are particularly weak or in those who have had failed surgeries, compensatory procedures have been used. These procedures involve placing a graft to reinforce the repair. Grafts are made of synthetic materials and biologic and autologous tissues. In assessing tension of the repair at the time of surgery, the pelvic floor surgeon should recall that the muscles of the pelvic floor are relaxed under anesthesia (general or spinal). Postoperatively, these muscles will contract along with the endopelvic fascia due to its own contractile fibers. Furthermore, scarring and postmenopausal atrophy may also cause further narrowing (Segal and Karram, 2002). Although the etiology of postsurgical dyspareunia is not fully understood, these factors should be kept in mind when repairing the pelvic floor. Pelvic floor reconstruction may be performed through a vaginal, open abdominal, laparoscopic approach or any combination of these techniques. Because POP is often mixed, surgery commonly involves a combination of repairs addressing any affected compartments. In addition, an anti-incontinence procedure may be performed at the same time. In comparing the vaginal approach with the abdominal approach there is a benefit with the vaginal approach in that complications and recovery time are less with the vaginal approach (Weber and Richter, 2005). The operative time is generally less with the vaginal approach, as is the hospital stay and recovery time (Morley and DeLancey, 1988; Shull, 1999). However, there is evidence that the abdominal approach is more durable compared with a vaginal technique (Benson et al, 1996). This fact should be considered in younger and more active patients. Over the past decade the definition of success has been expanded to include patient satisfaction and symptom improvement in addition to objective measures. Patients’ expectations and readiness to undergo surgery for POP have been shown to impact their satisfaction and how they perceive their improvement (Kenton et al, 2007). In the first article to examine patient-selected goals and surgical outcomes, Elkadry and coworkers (2003) found that patient satisfaction after surgery for POP correlated highly with achievement of self-described goals. In contrast, patient satisfaction correlated poorly with objective measures of surgical success. Dissatisfaction was highly associated with feeling unprepared for surgery. Furthermore, patients perceived the experience of routine postoperative events such as pain, hospital discharge with a catheter, constipation, urgency incontinence, and minor effects of anesthesia as surgical complications. These perceived complications were also associated with dissatisfaction. Additionally, they also demonstrated dissatisfaction despite high cure rates. Thus, patient perception of perioperative events and bother from recurrent symptoms equate largely to postoperative dissatisfaction even in the presence of high objective cure rates. It is vitally important to recognize that the absence of symptoms influences patient satisfaction greater than the elimination of anatomic prolapse. Kenton and associates (2007) evaluated patients with standardized counseling using a three-page handout on what to expect after surgery. Women who perceived they were fully prepared for surgery were more likely to be improved on the Patient Global Impression of Improvement (PGI-I) scale (Yalcin and Bump, 2003) and had lower postoperative scores on the Pelvic Organ Prolapse Distress Inventory (POPDI) (Barber et al, 2001) and Urogenital Distress Inventory (UDI) (Shumaker et al, 1994). Also, they found that objective measures of cure did not differ by preparedness. In conclusion, the preoperative surgeon-patient interaction is a key time that can be used to improve postoperative patient satisfaction. Therefore it is recommended to complete a thorough consent about the surgery, alternatives to surgery, the purpose of planned surgery (what it can and cannot accomplish), the benefits of surgery (what symptoms can be improved), the risks, and complications. In addition, it is useful to review what to expect while the patient is in the hospital, what to expect at home, and strategies on coping with a catheter both in the hospital and at home if needed (Kenton et al, 2007). In 2001 the Agency for Healthcare Research and Quality (AHRQ) identified perioperative interventions to improve patient safety (Shojania et al, 2001). These include asking the patient to recall and restate what she was told during the informed consent process, administering appropriate antibiotic prophylaxis to prevent postoperative infections, and implementing prophylaxis to prevent venous thromboembolism in patients at risk. The second most common source of nosocomial infections is infection at the surgical site (Bratzler and Houck, 2004). Current guidelines by the Centers for Medicare and Medicaid Services recommend that prophylactic antibiotics be administered within 60 minutes of incision time and discontinued within 24 hours of surgery (Centers for Medicare and Medicaid Services, 2008). The antibiotic chosen should be active against the likely infectious organisms to be encountered during the surgery performed. Although the American College of Obstetricians and Gynecologists has provided recommendations for antibiotic prophylaxis in patients undergoing hysterectomy there are no current guidelines addressing prolapse operations without hysterectomy (ACOG, 2009). Most favor the use of prophylactic antibiotics in reconstructive vaginal surgery, especially in surgery involving synthetic and biologic grafts. The risk of deep venous thrombosis (DVT) after major gynecologic surgery in patients not receiving prophylaxis is between 15% and 40% (Gutt et al, 2005). Risk factors include increasing age, previous venous thromboembolism, gynecologic malignancy surgery, and major pelvic reconstructive surgery. Waetjen and associates (2003) found that 42% of patients having surgery for POP were older than 60. The Centers for Medicare and Medicaid Services has endorsed a risk stratification presented by Geerts and colleagues (2004) that states that patients older than age 60 undergoing major surgery are at high risk for DVT. Without prophylaxis, high-risk patients have a 20% to 40% risk for calf DVT, a 2% to 4% risk for pulmonary embolism, and a 1% risk of fatal pulmonary embolism. They have recommended both anticoagulant-based and mechanical prophylaxis in patients at high risk for DVT. Specifically, heparin 5000 units three times a day and low-molecular-weight heparin with or without sequential compression devices are acceptable choices. Patients with advanced POP and incomplete bladder emptying may be taught to perform clean intermittent catheterization (CIC) preoperatively in the event that complete bladder emptying becomes a problem postoperatively. In postmenopausal women, local estrogen therapy with vaginal cream to treat atrophic vaginitis is started 6 weeks preoperatively. The administration of unopposed low-dose vaginally administered estrogen has been shown to be both safe and effective (Robinson and Cardozo, 2003). Also, local estrogens may increase vascularity and facilitate healing (Cervigni, 1996). The use of yellowfin (Allen, Acton, MA) or comparable stirrups, careful positioning of the patient in a neutral anatomic position, and avoiding leaning on or into the patient will minimize the risk of neuropathy, specifically to the lateral peroneal nerves. Although there has been an increased use of interposition graft materials in the area of pelvic floor reconstruction there are little controlled data to recommend their use. Graft materials have been used in an effort to improve outcomes of transvaginal prolapse repairs. The risk factors for failure of prolapse surgery include age, vaginal parity, smoking, deficient tissue quality, conditions that impair wound healing (diabetes mellitus and corticosteroid use), and conditions that stress the repair (chronic constipation, chronic obstructive pulmonary disease, and obesity). When examining the issue of failed prolapse surgery there are limitations. The definition of failure is not standardized, and many studies are short term, lacking controls. However, in spite of the limitations it is clear that failure of prolapse surgery is not uncommon. Some patients with POP have specific deficiencies in their tissues, which may predispose them to failure with traditional restorative procedures. Abnormal type I to type III collagen ratios have been identified, leading to a less organized collagen matrix (Falconer et al, 1998). Normal collagen protects fibroblasts from apoptosis, whereas abnormal fibroblasts are not protective (He et al, 2002). Decreased numbers and impaired function of fibroblasts have also been cited (Poncet et al, 2005). Overexpression of matrix metalloproteinases that break down extracellular matrix proteins have been demonstrated in women with prolapse (Jackson et al, 1996). These factors imply that these affected tissues may be less likely to respond to the dynamic forces placed on the female pelvic floor (Chen et al, 2004). As a result of these potential tissue disorders and inherent tissue weakness, graft interposition has been utilized with increased frequency. Although there is level I evidence supporting the use of synthetic graft materials for abdominal sacrocolpopexy (Davila et al, 2005) there is little evidence regarding the most appropriate usage of transvaginal mesh in prolapse repairs. The ideal prosthetic implant would be biocompatible, chemically inert, noncarcinogenic, mechanically strong, and sterile, have minimal allergic or inflammatory reaction, and avoid shrinkage and mechanical stress (Birch, 2005). In addition, the ideal implant should be readily available and affordable. Grafts may be categorized as either synthetic or biologic, and both graft types are used in pelvic floor reconstruction. Synthetic graft materials are usually classified as absorbable or nonabsorbable (permanent). Permanent graft materials are usually classified by pore size (macroporous, microporous, submicroporous, and combined) and by material structure (monofilament or multifilament). Biologic grafts are classified by source—autologous or heterologous—and further categorized as allografts or xenografts (Fig. 72–11). Figure 72–11 Classification of graft materials. (From Togami J, Krlin R, et al. Graft materials in prolapse surgery. AUA Update Series 2008;27[31]:294–303, with permission.) Graft incorporation of the tissues involves a foreign body response. Although many materials used for synthetic grafts are reported to be chemically and physically inert and nonimmunogenic (Davila et al, 2005; Deprest et al, 2006), none is biologically inert. Regardless of the material type, wound healing with a graft follows a stepwise cascade. Initially a biofilm is produced in response to tissue injury. Low-molecular-weight protein adsorption occurs quickly at the interface and does not require a cellular response. Complex proteins such as fibrinogen, immunoglobulins, and extracellular matrix proteins follow. If bacteria are incorporated into the biofilm at this point later wound healing can be affected (Deprest et al, 2006). These complex proteins undergo a conformational change to become more immunogenic, which leads to an inflammatory cascade by activating complement, binding antibodies, leukocytes, blood clotting, and fibrinolysis (Tang and Eaton, 1995). At this point the acute inflammatory response becomes chronic and granulation tissue forms. This involves fibroblasts, macrophages, giant cells, and, later, neovascularization and fibrosis. At this point the implant is mechanically stable. The amount of foreign body reaction is proportional to the surface area of the material exposed to the host. The degree of response and amount of tissue ingrowth is determined by the nature of the material, its structure, and the amount implanted for biologic grafts (Deprest et al, 2006). Ideally, host-tissue integration into the graft takes place, leading to long-term graft function after this biologic transformation. This illustrates an important concept in that the graft, biologic or synthetic, acts as a scaffold to provide a substrate to facilitate tissue ingrowth, rather than it functioning as a permanent mechanical support (Birch, 2005). Once a graft has been implanted into the human host, one of four processes may occur: (1) rejection in which there is severe and chronic inflammatory reaction around the implant, (2) degeneration (the result is necrosis from in-situ exothermic polymerization by the host), (3) encapsulation (minimal foreign body response in which there is fibrosis around the graft), or (4) incorporation into the human host tissue (a response in which there are inflammatory cells and giant cells leading to ingrowth of host tissues into the graft) (Williams, 1973). For long-term graft survival it appears that incorporation by the host through a process known as graft remodeling is necessary (Fig. 72–12). Figure 72–12 Host tissue ingrowth (process of graft remodeling) into polypropylene mesh. (From Woodruff A, Cole E, Scarpero H, et al. A histologic analysis of sling graft materials: a comparative study. Urology 2008;72:85–9, with permission.) Pore size affects host fibroblast infiltration, flexibility, and mechanical integration of the graft (Klinge et al, 2002). Large pore size has been shown to facilitate this process by enhancing host tissue infiltration and reducing the amount of inflammatory reaction with increased fibroblast infiltration. The large pores become integrated in a loose network of perifilament granulomas filled with fat, which maintains elasticity. After ingrowth of host tissue, a process of transformation occurs to complete the process of remodeling. Solid products or products with small pores (< 50 µm) induce a foreign body reaction that fills the entire pore. This promotes the bridging of the filaments to each other, thereby predisposing to encapsulation (Beets et al, 1996). Kaupp and colleagues (1979) described four histologic stages of the host to biologic graft implantation: Once the entire graft is infiltrated with host tissue the transformation process is complete. If this remodeling process characterized by ingrowth and transformation occurs before the graft substrate dissolves, long-term viability of the implant may be ensured. Although synthetic mesh material is a permanent substrate, the principles of tissue incorporation are necessary to prevent infection, extrusion, or erosion. Extrusion is thought to be secondary to exposed graft, which is attributable to localized infection, inadequate closure of the wound, or poor tissue quality (decreased vascularity or thickness) (Birch, 2005). Erosion is thought to be a chronic process related to host inflammatory response at the tissue-graft interface. Because of concerns regarding potential complications associated with synthetic grafts, some authors have turned to biologic grafts to circumvent these complications (Togami et al, 2008). Biologic grafts are categorized as autologous (patient serves as the donor), heterologous (same species, different individual [cadaveric]), and xenogenic (obtained from other species, typically porcine). The advantages of biologic grafts include in-vivo tissue remodeling, histologic similarity, and decreased propensity to elicit local complications (Silva and Karram, 2005). The donor sites for autologous grafts include rectus fascia, fascia lata, and vaginal epithelium, with the most common being rectus fascia and fascia lata. Autologous grafts are ideal in that they are well incorporated, pose no threat of disease transmission, and have minimal risk of encapsulation or rejection. The major drawback is the harvesting process, with increased morbidity, time, and potential for complications at the donor site. These factors are eliminated by using allografts and xenografts (Jarvis et al, 1985). Allografts are obtained from cadaveric donors and include dura mater, fascia lata, and dermis. Cadaveric fascia lata and dermis are used most commonly. A major concern when utilizing biologic materials is the potential for disease transmission. Choe and Bell (2001) demonstrated intact DNA in freeze-dried/irradiated cadaveric fascia lata and freeze-dried cadaveric dermal allograft. Fitzgerald and associates (2000) also examined irradiated freeze-dried or solvent dehydrated cadaveric fascia lata and found that it retained the donor human leukocyte antigen (HLA) class I and II antigens. Although extensive steps are taken to prevent infection exposure, risk of prion and human immunodeficiency virus transmission is estimated to be 1 in 1.7 million (Buck et al, 1989). To date there have not been any reports of disease transmission from grafts used for POP. The harvesting techniques of nonautologous biologic graft materials are standardized. In their review, Chen and colleagues (2007) described the process of allograft acquisition. Before allografts are harvested the donor undergoes serologic testing for hepatitis B and C, human immunodeficiency virus, and human T-lymphotropic virus type 1. Aseptic technique is used during harvest, and cultures are obtained at the time. The source animals for xenografts are specifically raised for medical purposes with production being strictly controlled by U.S. Food and Drug Administration (FDA) guidelines (Deprest et al, 2006). Materials available are derived from porcine subintestinal mucosa, porcine dermis, bovine dermis, and bovine pericardium, although the most commonly used are from porcine sources. These function as acellular collagen–based scaffolds to serve as a platform for host infiltration. Allografts and xenografts must undergo tissue processing before implantation, and, unlike harvesting, there is a variance in the processing techniques of these materials. This variance may affect the biologic and biomechanical properties of the grafts. There is no consensus as to which method should be used to optimize tissue properties. In some cases, processing is carried out to affect the long-term effects of the tissue to decrease graft breakdown and increase host tissue ingrowth. Sterilization is achieved by freeze-drying, solvent dehydration, and/or gamma irradiation. Lemer and associates (1999) demonstrated that freeze-dried cadaveric fascia demonstrated the least desirable characteristics when compared with autologous rectus fascia, cadaveric dermis, and solvent dehydrated cadaveric fascia lata. The freeze-dried cadaveric fascia demonstrated a reduced maximum load to failure and stiffness. In addition there was greater variability in the tissue’s strength and stiffness throughout its entire length. Cross-linking is another processing technique that can affect graft performance. This is done to delay reabsorption by collagenases (Badylak et al, 2002). Several methods are used, including aldehyde cross-linking and hexamethylene di-isocyanate (HMDI). Aldehydes are cytotoxic in high concentrations and may increase concentrations of gelatinases, which may actually increase the rate of degradation (Jorge-Herrero et al, 2001). In addition, aldehydes may cause calcification of the grafts, adversely affecting their function. Studies performed in rats have shown no graft mineralization with HMDI cross-linked dermal implantation at 2 years in contrast to aldehydes (Oliver, 1987). Local complications such as encapsulation may occur after the use of porcine dermis grafts (Cole et al, 2003). Graft fenestrations have been reported to enhance ingrowth and angiogenesis (Taylor et al, 2008). They may also decrease seroma formation and local complications. Porcine small intestine submucosa (Surgisis, Cook Medical, Bloomington, IN) is a non–cross-linked graft processed so that the complex extracellular matrix and natural growth factors are left intact (Cook Medical, 2007). There is histologic evidence that by 1 month the strength and histology of the graft is identical to native material and at 2 years it exceeds the strength of native tissue (Konstantinovic et al, 2005). The lack of in-vivo data limits the study of many biologic and synthetic grafts. Naturally it would be of benefit to demonstrate how the biomechanical properties of these materials are altered or remodeled by the host. Several animal models have been used to study grafts and meshes. In the rabbit model a free or pedicle flap of autologous rectus fascia decreased 37% in length, 63% in width, and 53% in tensile strength after implantation for 12 weeks. Neovascularization, minimal inflammation, and fibrosis was noted only along the permanent suture used to secure the graft (Fokaefs et al, 1997). In a rabbit model, freeze-dried, irradiated cadaveric fascia lata had a 90% decrease in tensile strength 12 weeks after implantation (Walter et al, 2003). There was variability in tensile strength from lot to lot and from grafts taken from different areas in the same lot. In an extensive rabbit study examining six different graft materials, tensile strength and stiffness of human cadaveric fascia and porcine xenografts decreased by 60% to 89%. Polypropylene (Prolene) mesh and anterior rectus fascia had no change in tensile strength from baseline (Dora et al, 2004). When comparing a tension-free vaginal tape (TVT) (Ethicon, Somerville, NJ) polypropylene sling to a cadaveric fascia lata sling in the rat model there was an advantage for the polypropylene sling in the breakload and maximum average load compared with cadaveric fascia lata (Spiess et al, 2004). There is a wide variation in the types of grafts available and tissue processing they undergo. It is unclear how this affects the performance of the grafts because there are little data comparing them. Before implantation, dermal allografts, solvent dehydrated fascia lata, and synthetic mesh have equal or higher tensile strength compared with autologous fascia. In some studies, freeze-dried grafts have a decreased tensile strength compared with similar grafts that have been solvent dehydrated. After implantation, autologous fascia and synthetic mesh seem to retain more of their tensile strength compared with allografts or xenografts (Chen et al, 2007). Synthetic mesh can be permanent or absorbable. Absorbable mesh has several desirable characteristics. It promotes postoperative fibroblast activity, has less infectious disease risk, is not rejected, and has not been reported to be harmful to the viscera. One disadvantage is that the resultant scar tissue may not be as strong as the native tissue it is reinforcing (Klinge et al, 2001). Classification of the synthetic meshes occurs by type of mesh (absorbable or nonabsorbable), pore size (macroporous or microporous), and filament type (monofilament or multifilament). Two types of absorbable meshes are available: polyglycolic acid (Dexon, Davis and Geck, American Cyanamid, Danbury, CT) and polyglactin 910 (Vicryl, Ethicon, Somerville, NJ). They differ in the duration in the tissues. Polyglactin 910 starts to hydrolyze by 21 days and loses its mechanical support by 30 days. Polyglycolic acid takes 90 days for absorption. The most important characteristic of a synthetic mesh is its pore size. Meshes are divided into macroporous (>75 µm) or microporous (<10 µm). Pore size is important from an infectious standpoint because it allows cellular elements such as macrophages and granulocytes to enter the mesh construct. In addition, it is important for tissue ingrowth with fibroblasts, blood vessels, and collagen fibrils (Amid, 1997; Deprest et al, 2006; Dwyer, 2006) (Fig. 72–13). Most bacteria are less than 1 µm; granulocytes and macrophages are greater than 10 µm in diameter, but 75 µm is the key size that allows the tissue ingrowth. Pore size also determines the flexural rigidity. The larger the pore size, the more flexible the mesh (Dietz et al, 2003). This property may impact local tissue trauma and erosive risk (Birch, 2005). (From Togami J, Krlin R, et al. Graft materials in prolapse surgery. AUA Update Series 2008;27(31):294–303.) Synthetic mesh can be monofilament or multifilament. Multifilament synthetics may have pore sizes that allow them to be classified as macroporous; however, between the fibers the size is less than 10 µm, owing to the way they are either woven or knitted. The spaces are small enough to allow bacteria into confines less than 10 µm, which carries a greater theoretical risk than a monofilament mesh. The construct of the mesh affects pore size, and the interstitial distance is measured between the synthetic fibers (Fig. 72–14). Woven mesh has small pore size and interstices, whereas knitted materials are able to assume a macroporous configuration and are flexible with high tissue conformity. Although there is no classification system specifically used for mesh in pelvic floor reconstruction, the Amid classification, which was originally used to describe mesh used in the treatment of abdominal wall hernias, is currently the standard (Amid, 1997). This classification emphasizes the importance of pore size and filament type (see Fig. 72–11). Type I promotes host defenses and tissue infiltration. Type II and type III meshes result in a greater foreign body response and are associated with greater rates of erosion (Julian, 1996; Debodinance et al, 1999). The most desirable synthetic for pelvic floor reconstruction are monofilament, macroporous mesh, most commonly polypropylene mesh. Kelly described his method of cystocele repair as a treatment for urinary incontinence in 1913. He emphasized the importance of repairing the pubocervical fascia with plication sutures to repair the central defect. This procedure eventually became known as the anterior colporrhaphy, and it corrects the central defect in the anterior compartment. However, anterior compartment defects are commonly combined central and lateral defects. Thus, an isolated anterior colporrhaphy is not recommended except in rare circumstances. In addition, the American Urological Association Female Stress Urinary Incontinence Clinical Guidelines Panel, in 1997, performed a meta-analysis and found that the failure rate of anterior colporrhaphy as a treatment for stress urinary incontinence (SUI) was close to 40% after 4 years of follow-up (Leach et al, 1997). As such, anterior colporrhaphy is not recommended as treatment for SUI. Table 72–2 Surgical Approach to Pelvic Organ Prolapse After placement of the patient in the dorsal lithotomy position an indwelling urethral catheter is placed and the bladder is kept drained throughout the procedure. Hydrodissection may be utilized to facilitate the dissection. A midline incision is made in the anterior vaginal wall, extending from the vaginal apex to the bladder neck. The incision should not extend to the urethra when a simultaneous midurethral sling is performed. Optimally the sling should be placed through a separate, suburethral incision. However, when performing a pubovaginal sling, it is desirable to create one incision that extends to the distal urethra and facilitates more precise sling location. The vaginal epithelium is dissected off the pubocervical fascia, and the dissection should be carried out sufficiently to delineate the defect. Usually, the incision should extend as far laterally as possible. Allis clamps or a self-retaining ring retractor may be utilized to provide optimal exposure. The bladder is then reduced with a finger or instrument, and this facilitates exposure and reapproximation of the pubocervical tissues. Once these tissues are identified, interrupted 2-0 delayed absorbable plication sutures are then placed from the bladder neck to the apex. The plication sutures are then tied. Care must be taken to avoid excessively deep suture placement to avoid ureteral injury. If the pubocervical fascia is too attenuated, a piece of allograft or mesh can be placed under the vaginal wall on one side to strengthen the repair and prevent suture pull-through (Chesson et al, 1999). After completion of the colporrhaphy, cystoscopy is performed after the administration of indigo carmine or methylene blue to inspect the bladder for any iatrogenic injury and to visualize urine effluxing from each ureteral orifice. Finally, the vaginal wall is trimmed and closed with a 2-0 absorbable suture. (From Nicholas DH. Cystocele. In Nichols DH, editor. Gynecologic and obstetric surgery. St. Louis: CV Mosby; 1993. p. 334–62.) In earlier studies, the reported cure rate of cystocele approached 95% (Table 72–3). In a large, retrospective study that included 299 patients with anterior vaginal wall prolapse, Porges and Smilen (1994) reported a recurrence rate of only 3%, with a mean follow-up of 31 months. Recurrence was defined as only symptomatic descent for which further surgery was performed or recommended. Also, in a prospective, randomized trial between Burch colposuspension versus anterior colporrhaphy, Colombo and colleagues (2000) reported a recurrence rate again of only 3% in the 33 patients who underwent anterior colporrhaphy. The mean follow-up was 5 years. All of the patients in both groups underwent concomitant hysterectomy and uterosacral ligament fixation. Recent series have reported recurrence rates for standard anterior colporrhaphy that approach 40% (Table 72–4). Recurrence in these series included asymptomatic grade 2 or stage 2 anterior compartment prolapse. Sand and associates (2001) reported on 161 women randomized to either anterior colporrhaphy with polyglactin suture or anterior colporrhaphy with a free polyglactin mesh inlay, which was placed underneath the trigone after plicating the pubocervical fascia. After 1 year of follow-up, the women randomized to polyglactin mesh had a failure rate of 25% compared with the 43% failure rate in those women who underwent the plication with suture alone (P = .02). Weber and colleagues (2001) compared anterior colporrhaphy reinforced with polyglactin mesh to traditional plication without tension using polydioxanone suture and “ultralateral” plication using both polydioxanone suture and tension. In this study, 114 women with symptomatic cystoceles (mostly stages 2 and 3 on the pelvic organ prolapse quantification [POP-Q] system) were randomized between the three groups. With a mean follow-up of 23 months there was no significant difference in failure rate (defined as stage 2 or greater on the POP-Q) between the three groups. The failure rate was 30% in those who underwent traditional plication, 46% in those who underwent ultralateral plication, and 42% in those who underwent traditional plication augmented with polyglactin mesh. Unfortunately, the study did not recruit enough women to achieve statistical power to detect differences between the groups. This is in contrast to an earlier paper by the same author that stated that anterior vaginal wall prolapse recurs after standard anterior colporrhaphy in 20% of patients (Weber and Walter, 1997). The Sand and Weber trials are not similar enough to do a meta-analysis. Yet, the high recurrence rates observed with anterior colporrhaphy illustrates the main concern regarding the durability of this procedure in most women with moderate- or high-grade anterior compartment defects. The most likely contributing factor is the concomitant presence of other compartmental defects. Reported complications from anterior colporrhaphy include de novo SUI (if the patient had SUI masked by a high-grade cystocele), de novo detrusor overactivity, postoperative urinary retention, incomplete bladder emptying, significant bleeding of more than 350 mL, wound infection, bladder or ureteral injuries, vaginal shortening, and dyspareunia. De novo detrusor overactivity can occur in 5% to 7% of patients after standard colporrhaphy (Raz et al, 1991). Yet, preexisting detrusor overactivity has been reported to resolve in up to 63% of patients after surgical repair of pelvic organ prolapsed (Nguyen and Bhattia, 2001). Urinary retention usually happens in cases during which an anti-incontinence procedure is performed. In most instances, the retention resolves after a brief period of CIC. If the retention persists and the patient had a concomitant anti-incontinence procedure, urethrolysis is often required. When impaired bladder emptying is due to preexisting hypocontractility, the patient may require chronic CIC or neuromodulation. Bladder or ureteral injuries are rare during standard anterior colporrhaphy. Bladder injuries can occur when perforation into the retropubic space occurs. Ensuring bladder drainage before perforating the endopelvic fascia may reduce this. Should an inadvertent bladder injury occur, a two-layer closure should be performed with absorbable suture. If the patient has a history of pelvic irradiation or if the repair quality seems tenuous, a labial fat pad interposition should be considered to prevent vesicovaginal fistula formation (Kreder, 1993). Cystoscopy after the administration of indigo carmine should be routinely performed with every anterior colporrhaphy. If blue-stained urine is not seen effluxing from either ureteral orifice, ureteral catheterization should be considered before taking down the plication sutures. In some cases the ureter is patent and an alternative reason will explain the lack of efflux. Appropriate steps must be taken to ensure ureteral patency if the ureter cannot be catheterized. This may include taking down the repair. In his first description of a vaginal paravaginal repair, George White (1909) described a cystocele repair that involved suturing the lateral sulci of the vagina to the white line of the pelvic fascia. White believed that the cause of a cystocele was weakness of the lateral vaginal attachments to the ATFP. As Kelly’s anterior colporrhaphy became the primary technique of cystocele repair for the next 70 years, White’s concept fell into disfavor. In 1976, Richardson and coworkers (1976) reintroduced the paravaginal defect repair, but they described the technique using an abdominal approach. Regardless of the approach the goal of a paravaginal repair is to repair a lateral compartment defect by reattaching the pubocervical fascia to the ATFP. A vaginal paravaginal repair may be combined with anterior colporrhaphy in patients with combined lateral and central defects. (From Mallipeddi PK, Steele AC, Kohli N, et al. Anatomic and functional outcome of vaginal paravaginal repair in the correction of anterior vaginal wall prolapse. Int Urogynecol J 2001;12: 83–88, with permission.) Shull and colleagues (1994) reported that 41 patients (73%) had no cystocele recurrence with vaginal paravaginal repair after a mean follow-up of 1.6 years. Of the 15 recurrences, only 4 developed to or through the hymen, and in these the prolapse was less than what was noted preoperatively. Elkins and associates (2000) reported an 8% lateral cystocele recurrence rate and a 22% central cystocele recurrence rate 0.5 to 3 years after vaginal paravaginal repair in 25 patients with grade 3 or greater anterior wall vaginal prolapse. Midline central defects were common, leading surgeons to perform joint repairs with an anterior colporrhaphy. Young and coworkers (2001) retrospectively evaluated 100 patients with symptomatic grade 2 to 4 combined central and lateral cystoceles. All 100 patients underwent both vaginal paravaginal repair and concomitant anterior colporrhaphy. With a mean follow-up of 11 months, the lateral cystocele recurrence rate was only 2% and the central cystocele recurrence rate was 22%. Mallipeddi and colleagues (2001) reported a 3% central cystocele recurrence 20 months after vaginal paravaginal repair in 35 patients with grades 2 to 4 anterior vaginal wall prolapse. Of the 21 patients with concomitant SUI, 12 (57%) had persistent SUI after the paravaginal repair. The paravaginal repair, like standard anterior colporrhaphy, is ineffective in the treatment of SUI. Finally, there are no studies comparing paravaginal defect repair with or without midline anterior repair to standard anterior colporrhaphy alone. Although complications after vaginal paravaginal repair are infrequent, they are significant. Young and associates (2001) reported 21 major complications, including three major intraoperative hemorrhagic complications. Two patients developed lower extremity neuropathy, one of whom was in the lithotomy position for a prolonged period of time. Elkins and associates (2000) reported a transfusion rate of 12%. Mallipeddi and coworkers (2001) reported 2 patients with vaginal abscesses that required drainage, 1 patient with retropubic hematoma who required reexploration, and 1 patient with bilateral ureteric obstruction that resolved after takedown of the repair and suture replacement. The incision and dissection into the retropubic space are performed in a similar manner as for the vaginal paravaginal repair. Once access to the pelvic sidewall is achieved, the bladder is retracted medially, and 2 to 3 sutures (permanent or delayed absorbable) are placed into the ATFP and/or obturator internus fascia, which will serve as adequate lateral fixation for the graft. A Capio Suture Capturing Device (Boston Scientific, Natick, MA) may expedite placement of these sutures (Fig. 72–17). Before fashioning the graft it is useful to estimate the distance between the proximal and distal ipsilateral sutures and the distance between the sutures on each side. These measurements serve to determine the dimensions of the graft material, which is trimmed to size. At this point, central plication sutures may be placed. The previously placed pelvic sidewall sutures are then brought out to the corresponding locations on the graft. The sutures are then tied, securing the graft to the pelvic sidewall. It is useful to leave the sutures long until after cystoscopy confirms patency of the ureters. The vaginal wall is trimmed and closed with a 2-0 absorbable suture. Vaginal packing is placed at the end of the case. Figure 72–17 Capio needle driver utilized to facilitate suture placement in the arcus tendineus fascia pelvis. (Photograph courtesy of Victor Nitti.) Nicita (1998) described the use of polypropylene mesh for the treatment of anterior vaginal wall prolapsed. He sutured the mesh to the anterior aspect of the ATFP on each side using 3-0 polypropylene sutures. With 2 years of follow-up, only 3 of the 44 patients (7%) had prolapse recurrence. The only patient who developed dyspareunia had vaginal mesh extrusion (exposure), and this was managed with mesh trimming and vaginal closure. de Tayrac and associates, in 2002, described placement of polypropylene mesh (Gynemesh; Ethicon, Somerville, NJ) into the retropubic space in a tension-free fashion without suture fixation. The lateral extensions of the mesh were placed in contact with the ATFP and anchored with a transobturator approach. A total of 48 women with grade 3 and 4 cystoceles underwent this procedure. After a mean follow-up of 18 months the authors reported an anatomic success rate of 97.9%. Vaginal mesh extrusion was noted in 8.3%. In a subsequent series with 87 women and with 24 months of follow-up, de Tayrac and associates again (2005) used GyneMesh and found that 77 women (91.6%) were cured of their symptomatic anterior vaginal wall prolapse, defined as POP-Q stage 0 or 1. Of the 7 patients with recurrent cystoceles, 5 were asymptomatic. Vaginal mesh extrusion was again noted in 8.3%, and the de novo dyspareunia rate was 16.7%. In a randomized controlled trial of 202 women with anterior vaginal wall prolapse, Hiltunen and coworkers (2007) compared anterior colporrhaphy (n = 97) to the same procedure reinforced with low-weight polypropylene mesh (n = 105). After 1 year of follow-up the addition of the polypropylene mesh to the anterior repair did statistically decrease the risk of recurrent stage 2 or greater anterior prolapse. Recurrence was noted in 7 of 104 women in the mesh group versus 37 of 96 women in the group with no graft (P < .001). However, there was no statistically significant difference in symptomatic outcomes (pelvic pressure, vaginal bulging, or difficulty with bladder emptying) between the groups. Importantly, 18 patients in the mesh group (17.3%) had vaginal extrusion of the mesh, although only 4 of the 18 patients were symptomatic and none required total removal of the mesh. In a prospective study of 98 patients with stage 3 and 4 cystoceles using the POP-Q, Rodriguez and associates (2005) attached a 5 × 5-cm piece of polypropylene mesh to the obturator internus fascia using 2-0 polyglactin suture to repair the paravaginal defect (Fig. 72–18). This mesh was placed after repair of the central defect with horizontal mattress sutures of 3-0 polyglactin. All patients underwent a distal urethral polypropylene sling regardless of any SUI symptoms. They reported an 85% anatomic success rate, defined as stage 0 or 1. No patient experienced urinary retention. Three patients who did not report SUI before the procedure developed mild de novo SUI after the procedure. Overall quality of life due to genitourinary symptoms improved from 4.7 (unhappy) to 1 (pleased) (P < .005). (From Rogriguez LR, Bukkapatnam R, Shah SM, et al. Transvaginal paravaginal repair of high-grade cystocele central and lateral defects with concomitant suburethral sling: report of early results, outcomes, and patient satisfaction with a new technique. Urology 2005;66[Suppl. 5A]:57–65.) Groutz and colleagues (2001) prospectively enrolled 21 women with grade 3 or 4 anterior vaginal wall prolapse to cystocele repair using solvent dehydrated cadaveric fascia lata (Fig. 72–19). The graft was anchored transversally between the bilateral arcus tendineus and the cardinal and uterosacral ligaments. Also, 19 of the 21 women underwent concomitant pubovaginal sling for either symptomatic or occult SUI. After a mean follow-up 20 months, none of the patients developed a recurrent cystocele. In addition, no patient developed new-onset dyspareunia, pelvic pain, or postoperative vaginal stenosis. (From Groutz A, Chaikin DC, Theusen E, et al. Use of cadaveric solvent-dehydrated fascia lata for cystocele repair—preliminary results. Urology 2001:58:179–83.) Gandhi and colleagues (2005) published a prospective randomized controlled trial comparing ultralateral anterior colporrhaphy with 0 polyglactin suture (n = 78) to the same colporrhaphy reinforced with cadaveric fascia lata (n = 76) in women with grade 2 or greater anterior vaginal wall prolapse. Previous surgery did not preclude participation. Although there was a trend toward improved anatomic outcome with graft use, the addition of the fascia lata patch to the anterior repair did not statistically decrease the risk of recurrent grade 2 or greater anterior vaginal wall prolapse (P = .23). Of the 39 total patients with recurrent prolapse, 26 (67%) were asymptomatic. Interestingly, concomitant transvaginal Cooper ligament sling procedures were associated with a decrease in recurrent grade 2 anterior vaginal wall prolapse (P < .0001). Kobashi and coworkers (2000) first described the cadaveric prolapse repair and sling procedure (CaPS) as combined treatment of both symptomatic cystocele and SUI. The procedure is performed transvaginally using a 6 × 8-cm piece of cadaveric fascia lata and bone anchors. In a noncomparative study of 251 patients with a mean follow-up of 2 years after CaPS, Frederick and Leach (2005) noted a symptomatic cystocele recurrence rate of 7%. Because the cured/dry rate for SUI was 56%, with most of the failures occurring beyond 1 year, the authors questioned the long-term efficacy of the sling portion of the CaPS. Cadaveric dermis was evaluated by Chung and colleagues (2002) in 19 patients with combined SUI and symptomatic grade 3 cystocele. The proximal portion of the 3 × 7-cm graft was sutured to the uterosacral/cardinal ligament complex to repair the central cystocele defect, and the distal portion of the allograft was used as the sling. Patients with lateral and paravaginal defects were excluded. The mean follow-up was 28 months. One patient developed a graft infection and underwent graft removal. Of the remaining 18 patients, 16 patients had no cystocele recurrence. Seventeen of the 18 patients (94%) were cured of their SUI, including 10 patients who had prior incontinence surgery. Clemons and coworkers (2003) described a vaginal paravaginal repair using cadaveric dermis in 33 women with recurrent stage 2 or with primary or recurrent POP-Q stage 3 or 4 cystoceles. The 3 × 7-cm graft was positioned over the bladder base and secured to the ATFP with four sutures of 2-0 braided permanent polyester bilaterally. The mean follow-up was 18 months. Objectively, 13 patients (41%) had recurrent stage 2 cystoceles; however, only 1 patient (3%) had a symptomatic recurrence. All of the objective failures were failures of point Aa on the POP-Q, implying that the anterior vaginal wall was well supported proximally but not distally. Eight women underwent a concurrent TVT sling, and the TVT sling did not prevent objective failure. The majority of clinical experience in the use of xenografts for pelvic organ prolapse has been with porcine dermis. Pelvicol (CR Bard, Murray Hill, NJ) is acellular cross-linked porcine dermis (Chen et al, 2007). Gomelsky and associates (2004) reported favorable, durable outcomes using a 6 × 8-cm piece of porcine dermis sutured laterally to the ATFP to correct high-grade cystoceles (Fig. 72–20). In a retrospective review of 70 patients, 61 patients (87%) had no cystocele recurrence after a mean follow-up of 24 months. Of the 9 patients (13%) with recurrent cystoceles, none was symptomatic. One patient had superficial vaginal wound separation, which was treated with conservative measures. (From Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ procedures. J Urol 2004;171:1581–4.) Meschia and colleagues (2007) published a prospective randomized controlled trial comparing anterior colporrhaphy with 0 polyglactin suture (n = 103) to the same colporrhaphy reinforced with a 4 × 7-cm piece of Pelvicol (n = 98) in women with stage 2 or greater anterior vaginal wall prolapse as defined on the POP-Q. After 1 year of follow-up, the women randomized to receive the Pelvicol graft experience graft failure 7% of the time, compared with the 19% failure rate in those women who underwent the anterior colporrhaphy with suture plication alone (P = .019). One patient who received the Pelvicol graft had vaginal extrusion of the mesh 1 month after surgery. Finally, there were no differences between the two groups in the incidence of postoperative dyspareunia. In a recent retrospective study, Handel and associates (2007)
Surgical Anatomy of the Pelvic Floor
Anatomy
Supporting Structures
Bony Scaffolding
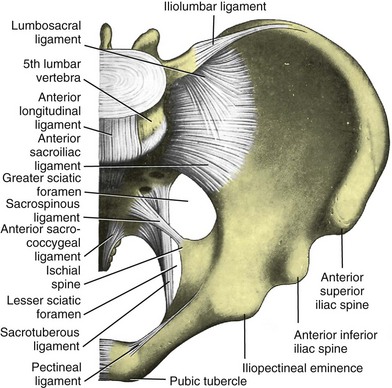
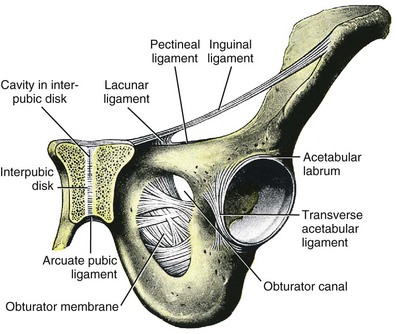
Muscular Supports of the Pelvic Floor
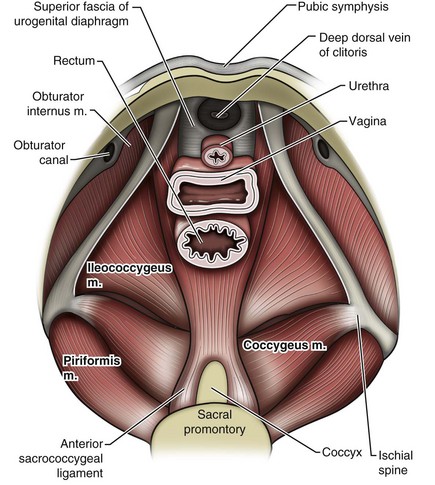
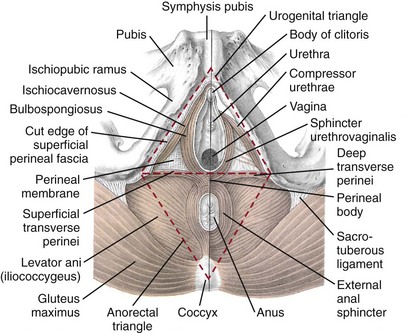
Endopelvic Fascia and Connective Tissue Supports
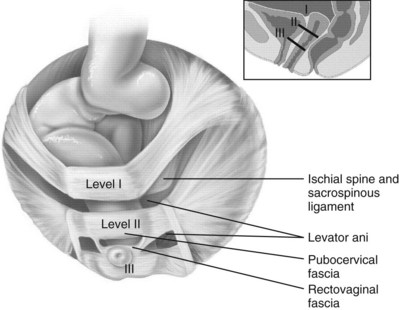
Support by Compartment
Anterior Compartment Supports
Apical (Middle) Compartment Supports
Posterior Compartment Supports
Pathophysiology of Pelvic Organ Prolapse: Surgical Correlation
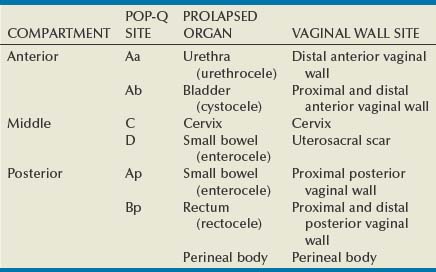
Site-Specific Defects by Compartment (Identification) (Table 72–1)
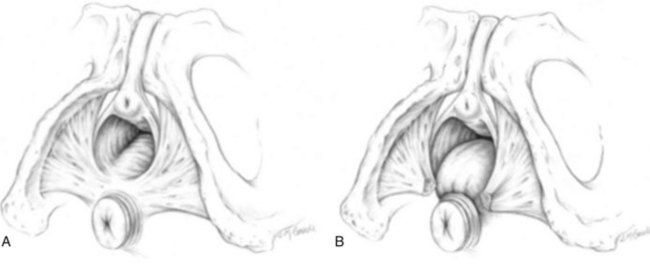
Anterior Compartment
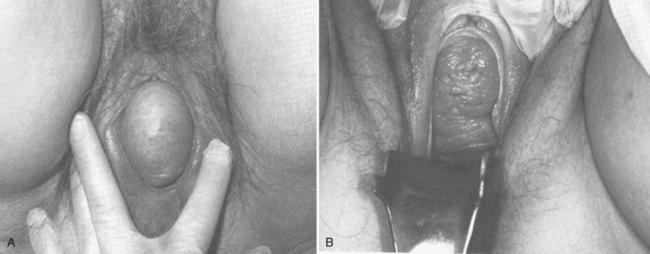
Apical Compartment
Posterior Compartment
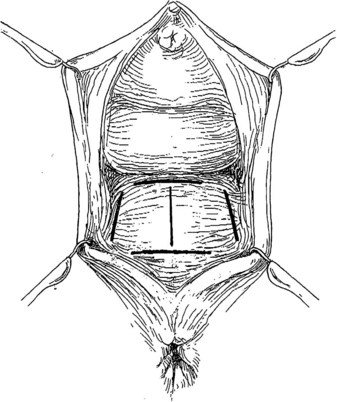
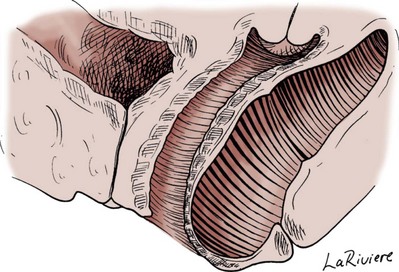
Preparing the Patient for Prolapse Surgery
Preoperative Counseling of Patient for Vaginal POP Surgery
Biologic and Synthetic Materials in Prolapse Surgery
Classification of Graft Materials
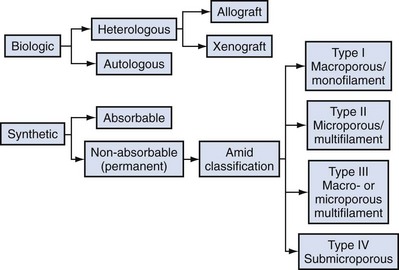
Basic Science: Graft-versus-Host Interaction
Clinical Application of Graft Materials
Biologic Grafts
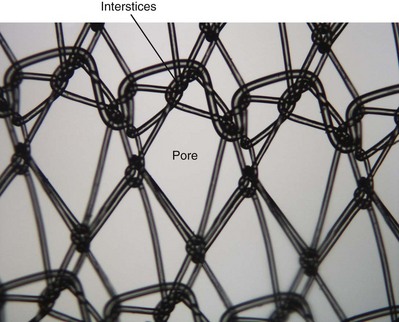
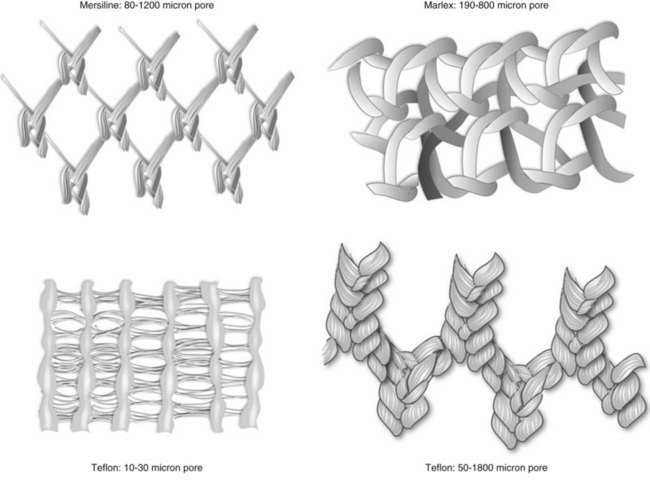
Synthetic Grafts
Surgical Management of Pelvic Organ Prolapse (Table 72–2)
Anterior Compartment
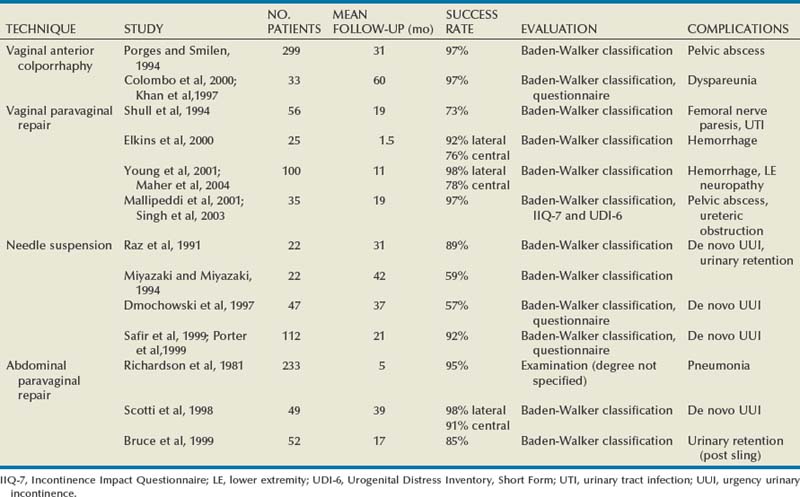
Anterior Colporrhaphy
POP-Q SITE
VAGINAL
ABDOMINAL
Aa
Retropubic urethropexy
Urethra
Ba
Bladder
C
Cervix/cuff
D
McCall culdoplasty
Cul-de-sac
Ap
Colpoperineopexy
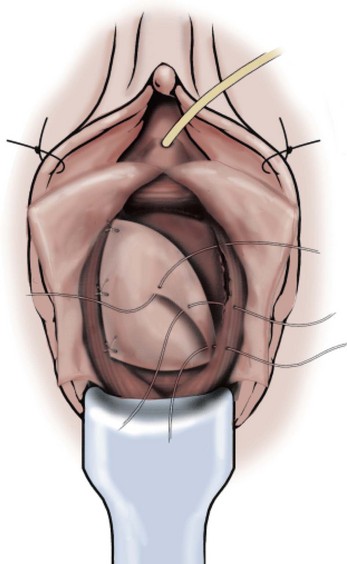
Technique (Fig. 72–15)
Results
Complications
Procedures for Lateral and Combined Defects
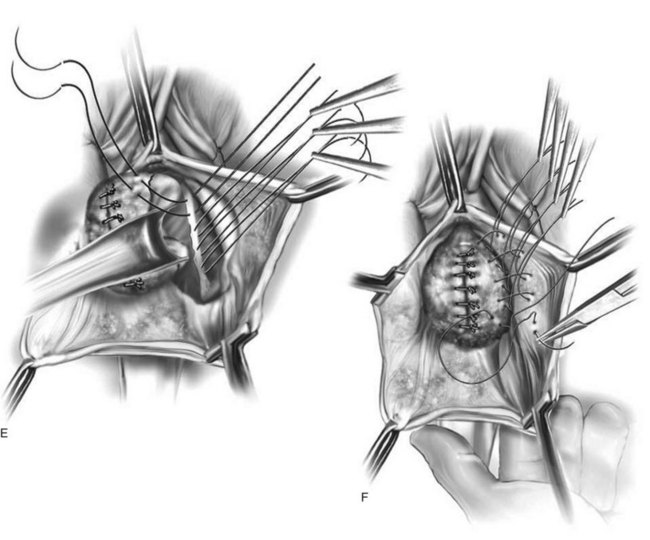
Vaginal Paravaginal Repair
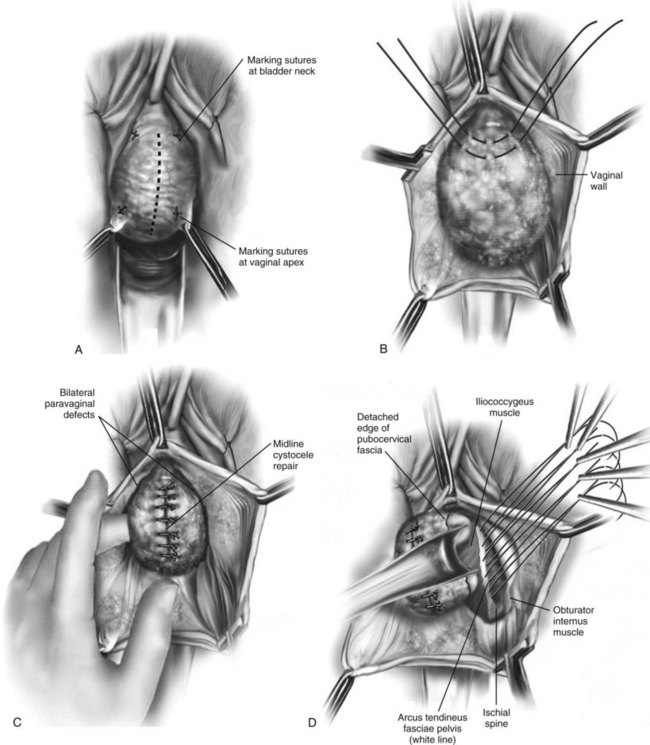
Technique (Fig. 72-16)
Results (see Table 72–4)
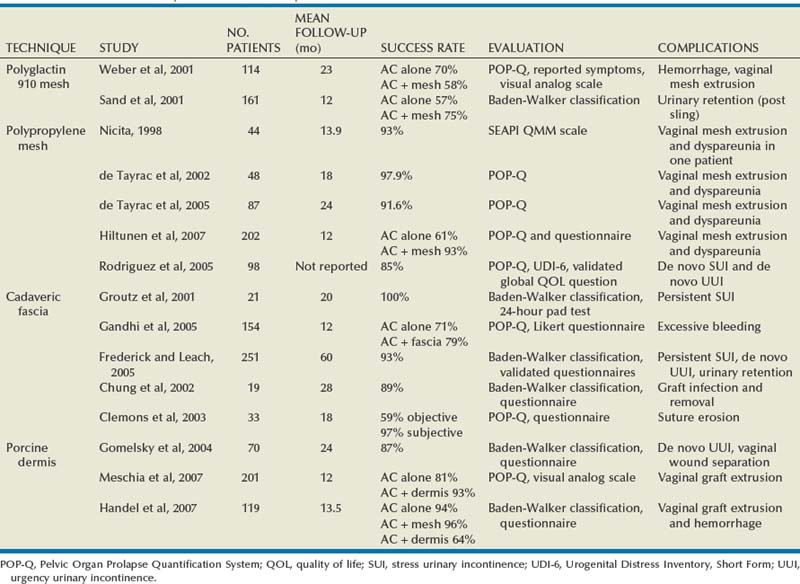
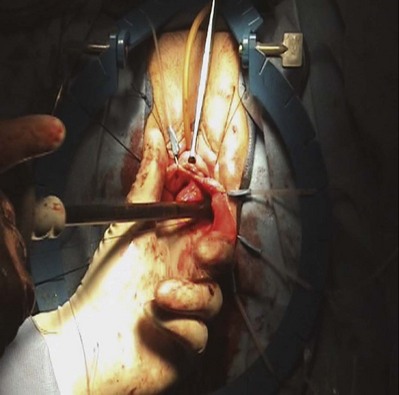
Anterior Compartment Repairs Utilizing Grafts
Technique
Results
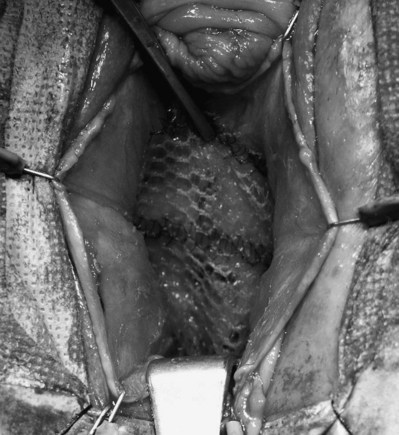
Polypropylene Mesh (see Table 72–4)
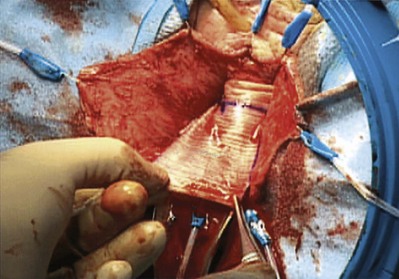
Cadaveric Fascia (see Table 72–4)
Porcine Dermis (see Table 72–4)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Vaginal and Abdominal Reconstructive Surgery for Pelvic Organ Prolapse
Stage 1 occurs over the first 7 days. This is marked by intense inflammation around the graft, which induces capillary proliferation, formation of granular tissue, and presence of giant cells.