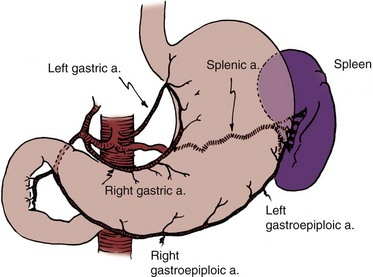
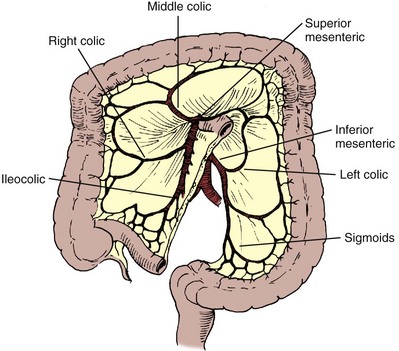
Douglas M. Dahl, MD, W. Scott McDougal, MD, MA (Hon) Please see the Expert Consult website for this section, including Figures 85-1 and 85-2. The stomach is a vascular organ that receives its blood supply primarily from the celiac trunk (Fig. 85–1). Three branches of the celiac axis give rise to the majority of the arterial supply of the stomach. The colon receives its blood supply from the superior mesenteric artery, inferior mesenteric artery, and internal iliac arteries (Fig. 85–2). The major arteries supplying the colon and rectum include the ileocolic, right colic, middle colic, left colic, sigmoid, superior hemorrhoidal, middle hemorrhoidal, and inferior hemorrhoidal arteries. These arteries anastomose one with the other to form the arc of Drummond and allow considerable leeway in mobilizing the colon. The middle colic artery arises from the first portion of the superior mesenteric artery and generally ascends the transverse mesocolon to the right of midline. The right colic artery usually arises just below the middle colic artery from the superior mesenteric artery and courses to the right colon. It may arise, however, from the ileocolic artery or directly from the middle colic artery. If it arises from the ileocolic artery, mobilization of the distal ascending colon is facilitated so that this portion of the colon can easily be brought into the deep pelvis. On occasion, however, it is necessary to sever the right colic artery at its origin to mobilize the distal portion of the ascending colon to the pelvis. This is particularly true if the right colic artery originates from the middle colic artery. The ileocolic artery is the terminal portion of the superior mesenteric artery and supplies the last 6 inches of ileum and ascending colon. The left colic artery arises from the inferior mesenteric artery, and then the inferior mesenteric artery gives off four to six sigmoid branches, the last of which becomes the superior hemorrhoidal artery. This anastomoses with the middle hemorrhoidal artery, a branch of the internal iliac artery, which in turn anastomoses with the inferior hemorrhoidal artery, the terminal branch of the internal pudendal artery. The middle sacral artery, which originates directly from the aorta, may supply the posterior aspect of the rectum. Please see the Expert Consult website for this section. The stomach, jejunum, ileum, and colon have unique properties, each of which has special advantages and disadvantages. The selection of the proper intestinal segment should be based on the patient’s condition, renal function, history of previous abdominal procedures, and type of diversion or substitution required. The stomach has been employed as a replacement for bladder, for augmentation cystoplasty, as a conduit, and for continent diversions (Bihrle et al, 1989; Abdel-Azim, 2003; DeFoor, 2003; Bissada, 2004). The advantage of the stomach over other intestinal segments for urinary intestinal diversion is that it is less permeable to urinary solutes, it has a net excretion of chloride and protons rather than a net absorption of them, and it produces less mucus. Urodynamically, it behaves like other intestinal segments. When it is used in urinary reconstruction, electrolyte imbalance rarely ensues in patients with normal renal function, although a hypochloremic metabolic alkalosis has been described. The incidence of bacteriuria has been reported to be as low as 25%, much less than the 60% to 80% incidence reported for ileal and colon segments. However, more recent data from our institution suggest that there is no difference in bacteriuria among any of the segments. The urine, which usually has a pH of 6 to 7, does not generally result in an increased incidence of peristomal skin problems. The authors have also noted that in bladder augmentation patients, there is little difference in urinary pH between gastric and ileal augmentations. Serum gastrin levels are generally normal or minimally elevated, depending on what portion of the stomach is used and how much (Leong, 1978; Adams et al, 1988). Although exclusion of the antrum from the gastrointestinal tract has not resulted in elevated serum gastrin levels and an ulcer diathesis clinically (Lim et al, 1983), antral exclusion experimentally results in elevated circulating gastrin levels, which may cause major intestinal ulcerative problems in the postoperative period (Tiffany et al, 1986). Rarely, severe ulcerative complications have been reported in cases that have employed stomach for urinary reconstruction (Reinberg, 1992; Tainio, 2000). Long-term H2 or proton-pump inhibition should be considered for these patients. When the antral portion of stomach is employed, reconstitution is generally by a Billroth I anastomosis. Complications with Billroth I gastroduodenostomy are well documented. The antrum should not be employed if the fundus is available. Early complications of the use of portions of the stomach for reconstruction include gastric retention due to atony of the stomach or edema of the anastomosis; hemorrhage, most commonly originating from the anastomotic site; hiccups secondary to gastric distention; pancreatitis as a consequence of intraoperative injury; and duodenal leakage. Delayed complications include dumping syndrome, steatorrhea, small stomach syndrome, increased intestinal transit time, bilious vomiting, afferent loop syndrome, hypoproteinemia, and megaloblastic or iron deficiency anemia. Postoperative bowel obstruction occurs with an incidence of 10% (2 of 21 patients) (Leong, 1978). Gastroduodenal and gastroureteral leaks have also been reported, occasionally resulting in a fatal outcome (Leong, 1978). The use of stomach for urinary intestinal diversion may be considered when the use of other intestinal segments in a patient with a decreased amount of intestine would result in serious nutritional problems. One advantage of using stomach segments in the patient with severe abdominal adhesions is that the area of the stomach is generally adhesion free and easily mobilized. Complications specific to the use of stomach include the hematuria-dysuria syndrome and severe metabolic alkalosis associated with respiratory distress in some patients (see “Metabolic Complications” later). The ileum and colon are used most often for urinary tract reconstruction and have been employed in all types of reconstructive procedures. The ileum is mobile and of small diameter, has a constant blood supply, and serves well for ureteral replacement and the formation of conduits. Loss of significant portions of the ileum results in nutritional problems because of lack of vitamin B12 absorption, diarrhea because of lack of bile salt reabsorption, and fat malabsorption. On occasion, the mesenteric fat is excessive, making mobility and anastomosis difficult. Also, the mesentery may be so short that it is difficult to mobilize the ileum into the deep pelvis. Postoperative bowel obstruction occurs in up to 10% of patients who have segments isolated from the ileum for urinary tract reconstruction (Varkarakis, 2006). As many as half of the obstructions occur in the early postoperative period (Schwarz and Jeffs, 1975). It has been a long-held tenet of elective intestinal surgery that bowel preparation is appropriate. The bacterial population in the stomach is relatively low, but in the remaining segments of the bowel including the jejunum, ileum, and colon, there are high bacterial counts. Early studies had suggested that bowel anastomoses in the patients whose intestinal tract had not been prepared before surgery had increased wound infection rates, increased intraperitoneal abscesses, and an anastomotic dehiscence rate greater than in those patients who have had proper bowel preparation before surgery (Irvin and Goligher, 1973; Dion et al, 1980). Other studies showed that mechanical preparation resulted in collapsed bowel at the time of surgery, which was shown to reduce the incidence of anastomotic leaks (Christensen and Kronborg, 1981). Studies have recently begun to question the widely held belief that bowel preparation is mandatory. In meta-analyses of randomized clinical trials of anastomotic leakage during colon and rectal surgery, researchers found that there was no support for the conclusion that bowel preparation reduces anastomotic leak rates and other complications (Guenaga et al, 2003; Slim, 2009). There are suggestions that mechanical bowel preparation may actually increase the rate of anastomotic leakage and wound complications (Guenaga et al, 2005). In experimental animals, it has been shown that an anastomosis with vascular compromise at the anastomotic line, which would normally result in perforation, heals if the bowel has been properly prepared with antibiotics. Also, solid feces may place strain on the anastomosis in the early phase of healing and result in ischemia with subsequent perforation. Complications that result from bacterial contamination are a major cause of morbidity and mortality in patients undergoing urologic procedures. Infectious complications after radical cystectomy that are a direct result of fecal contamination may occur in 18% to 20% of patients who undergo radical cystectomy and include wound infections, peritonitis, intra-abdominal abscesses, wound dehiscence, anastomotic dehiscence, and systemic sepsis (Bracken et al, 1981). More recent series suggest that current management practices appear to have made a substantial improvement with perioperative infectious complications of 7% (Stein et al, 2004). In another contemporary series of radical cystectomy with continent or ileal loop urinary diversion in 167 patients, there was an infection complication rate of 7.2% (Mansson et al, 2003). A 5.2% rate of infectious complications was reported in another contemporary series (Cookson et al, 2003). A mechanical bowel preparation reduces the total number of bacteria but not their concentration. Thus the same number of organisms is present per gram of fecal content (Nichols et al, 1972). Therefore spilling enteric contents during the procedure may be less likely with the mechanically prepared bowel because there is less of it to spill; however, once spilled, cubic centimeter for cubic centimeter, the inoculum is the same as if the bowel had not been prepared. Recent analysis has suggested, however, that there may in fact be an increase in bacterial contamination in patients who have undergone bowel preparation (Fa-Si-Oen et al, 2005). Conventional bowel preparations commonly used in the past tended to exhaust the patient and exacerbate nutritional depletion because they generally required a 3-day preparation period of insufficient calorie intake (Table 85–1). The use of elemental diets has been advocated to clean the colon of feces while not compromising the nutritional status of the patient. Unfortunately, they have not proved useful because the elemental diets do not empty the colon of feces, and they do not reduce the bacterial flora (Arabi et al, 1978). In an attempt to reduce the time required for intestinal preparation and to obviate low-calorie intakes, whole-gut irrigation has been used. Originally, whole-gut irrigation was performed by placement of a nasogastric tube (NGT) into the stomach and infusion of 9 to 12 L of lactated Ringer solution or normal saline during a several-hour period. These fluids were subsequently replaced with 10% mannitol, which was equally successful in ridding the bowel of its fecal content; however, the mannitol served as a bacterial nutrient and thereby facilitated microbial growth (Hares and Alexander-Williams, 1982). These solutions have largely been replaced by a polyethylene glycol–electrolyte solution. Whole-gut irrigation may be exhausting to the patient and may, in fact, result in a fluid gain, particularly when either saline or mannitol is used. Whole-gut irrigation is contraindicated in patients with an unstable cardiovascular system, patients with cirrhosis, patients with severe renal disease, patients with congestive heart failure, or those with an obstructed bowel. Whole-gut irrigation has been found to be no more effective than conventional preparations in reducing wound infections and septic complications (Christensen and Kronborg, 1981), even though there is a reduction of aerobic flora compared with the conventional preparations (van den Bogaard et al, 1981). The advantages of the whole-gut irrigation are that it gives the patient dietary freedom, there is a short preparation time, and it eliminates the enema. Its disadvantages are that it may result in the patient’s exhaustion, it is rather rigorous, and it does result on occasion in fluid overload. The polyethylene glycol–electrolyte lavage solution (GoLYTELY or the more palatable NuLYTELY) is an effective lavage agent in preparing the gut for elective colon and rectal surgery, as well as for urologic surgery in which bowel is used. For the adult, 20 to 30 mL/min or approximately 1 to 1.5 L/hr for 3 hours is given either orally or through a small-caliber NGT placed into the stomach. If it is taken by mouth, it is better tolerated if the solution is chilled. The administration of GoLYTELY is stopped when the rectal effluent is clear and there is no particulate matter in it or when 4 L of fluid has been given. This preparation in the adult has been as effective as conventional preparations. The septic complications with its use are approximately 4%. An inadequate preparation occurs in 5% of the patients using this modality (Wolff et al, 1988). For children, even those younger than 1 year, GoLYTELY may be used at a rate of 20 to 40 mL/kg/hour and given until the rectal effluent is clear and free of particulate matter (Tuggle et al, 1987). Metoclopramide (Reglan), 10 mg, in adults is often given simultaneously to control nausea. Bowel preparation can increase metabolic complications and cause electrolyte disturbances, which could affect surgical care. Caution must be exercised in elderly and debilitated patients receiving sodium phosphate preparation; the sodium phosphate preparation has been shown to cause significant derangements in potassium, calcium, and phosphorus levels in frail individuals (Beloosesky et al, 2003). Phosphate nephropathy has recently been recognized as a serious complication of oral sodium phosphate (OSP) bowel preparation (Markowitz, 2005). Caution should be used in prescribing OSP to patients with underlying renal insufficiency or treated with nephrotoxic medications. The only study in postsurgical complications comparing sodium phosphate with polyethylene glycol found no significant difference in complication rates (Oliveira et al, 1997). One study suggested that polyethylene glycol is better tolerated by elderly patients and causes less disruption in potassium and sodium levels (Seinela et al, 2003). Oral electrolyte solution rehydration may prevent some of the complications of bowel preparation (Tjandra and Tagkalidis, 2004). A number of studies have questioned the efficacy of mechanical bowel preparation. Some have suggested that a limited mechanical bowel preparation is all that is necessary; others have questioned even the need for a mechanical bowel preparation. In one study, 2 L of polyethylene glycol plus metoclopramide was compared with the administration of 4 L of polyethylene glycol solution. There was no difference in surgical complication rate or the extent to which the bowel was clean (Grundel et al, 1997). In another study, when 4 L of polyethylene glycol was compared with 90 mL of sodium phosphate, there was no significant difference in surgical complication rate (Oliveira et al, 1997). Two meta-analyses have found that there is an increased anastomotic dehiscence rate with preoperative mechanical bowel preparation (Wille-Jorgensen et al, 2003; Bucher et al, 2005). Polyethylene glycol may be the agent responsible for the increased rate of complications, but other preparation strategies have not been adequately analyzed (Slim et al, 2004). A recent meta-analysis of randomized trials comparing mechanical bowel prep to no bowel prep before elective colorectal surgery found no difference between the groups for anastomotic leakage, abdominal abscess, or wound sepsis (Slim, 2009). No study has adequately addressed the issue of the need for debulking of the intestine before laparoscopic approaches to intestinal surgery. It is extremely important to note that in these studies, the administration of intravenous antibiotics was crucial in keeping the complication rate low. Moreover, it is important to note that in these studies there was limited exposure of the intestine as the patients underwent elective bowel resections, unlike urologic procedures in which long segments of the intestine are opened or interposed in a urinary tract that is normally free of fecal contents. There has been considerable recent controversy as to whether the addition of antibiotics in elective colon and small bowel surgery reduces mortality and morbidity significantly. The long-held practice of mechanical and oral antibiotic bowel preparation dates to the 1970s. In one study, the septic complication rate was reduced from 68% in the control group to 8% in the antibiotic group (Washington et al, 1974). Most series, however, report a lesser incidence of reduction in wound infection, generally from 35% without antibiotics to 9% with their use (Clarke et al, 1977). Others have suggested that the mortality rate drops from 9% to 3% with the use of antibiotics (Baum et al, 1981). It is clear that the use of antibiotics protects vulnerable bowel in that it may allow the tenuous anastomosis to survive. Other studies, however, have shown that without the use of oral antibiotics in mechanically prepared bowel in elective surgery, the septic complication rate is comparable with those studies using antibiotics and the rate of Clostridium difficile colitis was lower without oral antibiotics (Wren, 2005). In the presence of a bowel obstruction, however, oral antibiotics are of little value because they do little good in sterilizing the bowel. The disadvantages of antibiotics include postoperative increase in the incidence of diarrhea; pseudomembranous enterocolitis; theoretical increased incidence of tumor implantation at the suture line that is not germane to urologic surgery; monilial overgrowth resulting in stomatitis, thrush, and diarrhea; and, with prolonged use, malabsorption of protein, carbohydrate, and fat. The antibiotics most commonly used for bowel preparation include kanamycin, which is the best single agent; neomycin and erythromycin base; and neomycin and metronidazole (Table 85–2). With an appropriate antibiotic preparation, enteric organisms are reduced to 102 per gram of feces (Nichols et al, 1972). A contemporary randomized trial of patients undergoing elective colonic surgery found the lowest fecal bacterial concentrations when patients had preoperative mechanical bowel preparation, oral neomycin, and supplemental “synbiotic” treatment (to provide benign flora to the intestine). No difference in clinical infections was seen between patients prepared with or without oral antibiotic bowel preparation (Reddy, 2007). Perioperative intravenous antibiotics appear to be the most important means of preventing infectious complications of intestinal surgery. Systemic antibiotics must be given before the operative event if they are to be effective. Ideally, antibiotics should be given between 1 and 2 hours before the start of surgery (Classen et al, 1992). They appear to be most effective against the anaerobic flora and apparently reduce the complications caused by these organisms (Dion et al, 1980). Perioperative systemic antibiotics, when added to the oral regimen, reduced the septic complication rate from 15% to 20% to half that rate in several series (Hares and Alexander-Williams, 1982; Gottrup et al, 1985). Other studies, however, have shown no effect of systemic cephalosporin, for example, in reducing septic complications (Wolff et al, 1988). If perioperative antibiotics are given, they should be effective against anaerobes because it is complications from these organisms against which perioperative antibiotics appear to be particularly effective. Third-generation cephalosporins have been advocated as an appropriate systemic antibiotic. Other recent studies support the use of both oral and systemic antibiotic prophylaxis before intestinal surgery (Lewis, 2002). It is clear that preoperative antibiotics reduce postoperative complications. Most agree that preoperative intravenous antibiotics are important, and many advocate discontinuing the use of oral antibiotics as the incidence of C. difficile diarrhea is increased and there appears to be no advantage provided preoperative intravenous antibiotics are given within an hour of the operative event (Wren et al, 2005). Antibiotic bowel preparations may result in diarrhea and pseudomembranous enterocolitis. Pseudomembranous enterocolitis is the more severe form of a spectrum of diarrhea. Clinically, this occurs after a bowel preparation in the postoperative period and is heralded by abdominal pain and diarrhea usually in the absence of fever or chills. As the symptoms and infection become more severe, systemic toxicity supervenes. These patients can develop a toxic megacolon, and if this occurs, the mortality may exceed 15% to 20%. Historically, pseudomembranous enterocolitis was thought to be due to staphylococcus, but there was, in fact, little evidence to support that organism as the etiologic agent. It is now clear that C. difficile plays a significant role in the majority of cases. C. difficile elaborates at least two toxins that cause diarrhea and enterocolitis. C. difficile does not invade the bowel, and it is not normally a significant inhabitant of the fecal flora. It is held in check by other bacteria that inhibit its growth. Thus antibiotics destroy the bacteria that inhibit the growth of C. difficile and thereby allow it to flourish. The toxin produces a diffuse inflammatory response with cream-colored plaque formation, erythema, and edema of the bowel wall. On microscopic examination, the villi appear to be intact and there is a polymorphonuclear leukocyte infiltrate of the submucosa (Bartlett, 2002). As the disease progresses, large areas of mucosa may slough and areas of the bowel are denuded of their mucosa. The lesions may involve the colon, in which case it is called pseudomembranous enterocolitis, or the small bowel, in which case it is called pseudomembranous enteritis, or they may involve both. The diagnosis is suspected by the symptoms or endoscopy and confirmed by culture of the organism or identification of its toxin. Because culture takes a prolonged time, it is more expeditious and therefore clinically useful to confirm the diagnosis by identifying the toxin produced by C. difficile. Once the diagnosis has been made, treatment involves the administration of vancomycin or metronidazole and discontinuance of other antibiotics that the patient is receiving. Vancomycin or metronidazole is effective in most cases. Rarely, toxic megacolon supervenes, requiring subtotal colectomy as a lifesaving procedure (Chang, 1985). Factors that significantly contribute to anastomotic breakdown include poor blood supply, local sepsis induced by fecal spillage, drains placed on an intra-abdominal anastomosis, and anastomosis performed in irradiated bowel. Poor blood supply and local sepsis cause ischemia. Drains placed on the anastomosis increase the likelihood of an anastomotic leak, and an anastomosis performed in irradiated bowel is more likely to result in an anastomotic failure than one performed in nonirradiated tissue. The importance of careful technique and adherence to these principles is emphasized by the fact that in one series of urinary intestinal diversion, 75% of the lethal complications that occurred in the postoperative period were related to the bowel. Eighty percent of these patients had received radiation before the intestinal surgery (Mansson et al, 1979). Intestinal anastomoses may be performed with use of sutures or staples. Properly performed, both have similar complication rates (Catena, 2004). In selected circumstances, however, one method may have advantages over the other. Because there is a high rate of stones that form on surgical staples (Woodhouse, 2004), absorbable suture should be used for intestinal segments that are exposed to urine (e.g., suturing intestine to renal pelvis or bladder, closing the proximal end of a conduit [Costello and Johnson, 1984], and forming an intestinal pouch for urine). A 3-0 silk holding suture is placed on the mesenteric border just beneath the Allen clamps traversing both segments to be anastomosed, and a second suture is placed on the antimesenteric border similarly just beneath the Allen clamps (Fig. 85–3). It is important that the mesentery is cleaned sufficiently so that these sutures are placed in the serosa under direct vision. A row of silk sutures is placed 2 mm apart between the two holding sutures. This is accomplished by rotating the two Allen clamps away from each other, thus apposing the serosal surfaces. Sutures must traverse the muscularis but should not traverse the full thickness of the bowel. After all sutures have been placed, each is tied and the tails of all the sutures are cut, except those at each end; these are used as holding sutures. The Allen clamps are removed, and hemostasis is achieved, if necessary, with the light application of electrocautery. A 3-0 double-ended chromic intestinal suture is placed in the posterior suture line through all layers and tied to itself. Each end of the suture is then run in a locking fashion away from the midpoint until the mesenteric and antimesenteric borders are approached. As the lateral aspects of the bowel are approached, the suture is converted to a Connell suture (Fig. 85–4), which proceeds onto the anterior bowel wall. The sutures meet anteriorly in the midline and are tied together. The anterior serosa is then apposed with interrupted 3-0 silk sutures. The noncrushing occlusive clamps are removed, and the mesentery is closed with interrupted 3-0 silk sutures. The single-layer anastomosis for reapproximating bowel is an excellent technique with a low complication rate, that is, a 0.2% anastomotic leakage rate compared with an 8.4% anastomotic leakage rate for a stapled anastomosis in one large series (Leslie and Steele, 2003). The mesenteries of the two segments of bowel to be anastomosed are aligned, and a 3-0 silk suture is passed through the seromuscular layers of both segments on the mesenteric side; a second suture is similarly placed on the antimesenteric side. The mesenteric suture is tied, and the antimesenteric suture is left untied. The Allen clamps are removed, and hemostasis is achieved with light electrocautery. The critical point of the anastomosis, where most leaks occur, is at the mesenteric border. Leaking generally occurs because the sutures are placed carelessly or the serosa has not been cleaned of mesentery sufficiently so that the sutures are placed through it under direct vision. Because this mesenteric border is the critical area, it is approached first. Two 3-0 silk sutures are placed through the full thickness of the bowel on either side of the mesenteric holding suture. These sutures are placed in such a way as to include more serosa than mucosa, thus causing inversion of the suture line (Fig. 85–5A). Some prefer to use a Gambee stitch at this point, which involves placing the suture through the full thickness of the bowel followed by traversing a small segment of mucosa of each segment of bowel before exiting through the full thickness of the bowel of the other segment (Fig. 85–5B). The two bowel sutures on the mesenteric border are tied, with care taken to invert the suture line, thus apposing serosa. Then 3-0 silk sutures are placed 2 mm apart, both on the anterior and posterior walls, inverting the suture line, thus apposing the serosa of the two bowel segments to each other. On approaching the antimesenteric holding suture, several sutures are placed before all are tied. A patent anastomosis is confirmed by feeling the annulus with the thumb and forefinger as described previously. The transected end of the colon is closed in the following manner (Fig. 85–6). A 3-0 silk suture is placed beneath the Allen clamp on the mesenteric border, and a second suture is placed on the antimesenteric border. These are tied. A 3-0 chromic suture is placed beneath the clamp in a horizontal mattress fashion. Beginning at the mesenteric border, it is tied to itself and the horizontal mattress suture is placed until the antimesenteric border is reached, at which point the suture is again tied to itself. The clamp is removed, and an over-and-over suture is performed with the same chromic suture throughout the full thickness of the bowel until returning to the point of origin (i.e., the mesenteric border is approached). At this point, the suture is again tied to itself. The suture line is buried by approximating the serosa on each side with interrupted 3-0 silk sutures placed 2 mm apart. Our preference is to close the end of the colon similarly to the way one closes the proximal end of a conduit. After a 3-0 silk suture is placed through the serosa on the antimesenteric and mesenteric sides, the clamp is removed and a 3-0 chromic suture is placed through all layers at the mesenteric and antimesenteric end. A Connell suture is used—the two chromic sutures meet in the middle and are tied together. Seromuscular sutures of 3-0 silk are placed to appose the serosal margins. The mesenteries are aligned, and the ileal serosa is sutured with interrupted 3-0 silk sutures to the colonic serosa 2 mm below a taenia (Fig. 85–7). The taenia is incised the length of the diameter of the ileum adjacent to it. As described earlier for the two-layer anastomosis, a 3-0 double-ended intestinal chromic suture is placed through all layers of the colon and ileum in the midpoint of the posterior wall and run in a locking fashion laterally to either side of the incision in the taenia. At the most lateral border, the suture is converted to a Connell suture and the anterior wall is closed. Seromuscular sutures of 3-0 silk placed from ileum to colon bury the anterior suture line. The mesentery is reapproximated. A 3-0 silk suture is placed on the mesenteric border of the ileum and colon (Fig. 85–8). A second 3-0 silk suture is placed on the antimesenteric border of the colon immediately beneath the Allen clamp. The other end of the suture is placed on the antimesenteric border of the ileum at a distance proximal to the Allen clamp so that the serosal lengths between the two sutures of both ileal and colon segment are equal. Thus an equal amount of ileal serosa is applied to the length of colonic serosa bordering the severed end of bowel. In the seromuscular layers of ileum and colon, 3-0 silk sutures are placed 2 mm apart, thus apposing the serosa of the ileum to the colon. The Allen clamps are removed. Hemostasis is achieved, and the antimesenteric border of the ileum is incised to the level of the most proximal suture in the ileum. Thus the bowel lumens are now of identical size. With a 3-0 chromic double-ended intestinal suture, the posterior row is run in a locking fashion, laterally converting to a Connell suture, and the anterior row is completed. Seromuscular sutures of 3-0 silk bury the anterior suture line. Figure 85–8 Anastomosis of discrepant-sized bowel. A seromuscular suture of 3-0 silk is placed adjacent to each end of the lumen on the mesenteric side. A second 3-0 silk seromuscular suture is placed adjacent to the lumen on the colon and on the antimesenteric border proximal to the cut end of the small bowel at a distance sufficient that when the antimesenteric border is incised, the lumens are the same size. Interrupted seromuscular sutures of 3-0 silk are then placed at 2-mm intervals between the two holding sutures. The small bowel is opened on its antimesenteric border until the opening in the small bowel is the same size as the opening in the colon. A 3-0 chromic suture is placed through all layers, tied to itself, and run laterally in a running locking fashion. At the borders, it is converted to a Connell suture, thus inverting the anterior margin. The anastomosis is completed with interrupted horizontal mattress 3-0 silk sutures that bring the seromuscular layers together anteriorly. This is similar to the closure depicted in Figure 85–3. The theoretical benefits of a stapled anastomosis are that it provides for a better blood supply to the healing margin, there is reduced tissue manipulation, there is minimal edema with uniformity of suture placement, a wider lumen is constructed, there is greater ease and less time involved in performing the anastomosis, and the length of postoperative paralytic ileus is reduced. When they are placed in intestine through which urine traverses, however, stapled anastomoses employing nonabsorbable staples frequently cause stone formation and should be avoided (Costello and Johnson, 1984; Woodhouse, 2004). The TA (transverse anastomosis) stapled anastomosis everts the suture line. Because staples close in a B and do not crush the tissue, theoretically they prevent ischemia at the suture line. This may be obvious when a staple line is used to transect the bowel and bleeding continues to occur. The bleeding points may be lightly electrocoagulated or tied off with fine absorbable suture. Stapled bowel anastomoses have been shown to be as efficacious as hand-sewn anastomoses because both have similar complication rates. They usually require less time to perform when the techniques are properly learned, but for prolonged procedures, they save little if any time when the length of time for the whole procedure is taken into account. In a large prospective, randomized trial in which a two-layer closure was compared with a staple closure, it was found that the complication rate was the same, but the time required to complete the stapled anastomosis was 10 minutes less than that for the hand-sewn anastomosis; when the total operative time was compared between the two, it was the same (Didolkar et al, 1986). A comparison of complications between sutured and stapled anastomoses reveals a leak and fistula rate of 2.8% for stapled and 3% for sutured anastomoses (Chassin et al, 1978). The clinically significant leak rate, however, is only 0.9% (Fazio et al, 1985). A 4.5% incidence of stapled anastomotic leakage has been reported during ileal conduit construction (Costello and Johnson, 1984).
Surgical Anatomy
![]()
Stomach
Colon
Selecting the Segment of Intestine
![]()
Bowel Preparation
Mechanical Bowel Preparation
Antibiotic Bowel Preparation
Diarrhea and Pseudomembranous Enterocolitis
Intestinal Anastomoses
Types of Anastomoses
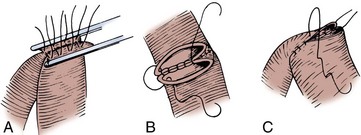
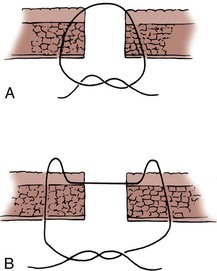
Enteroenterostomy by a Two-Layer Suture Anastomosis
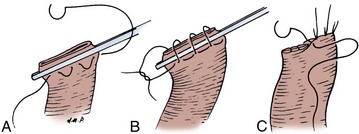
Enteroenterostomy by a Single-Layer Suture Anastomosis
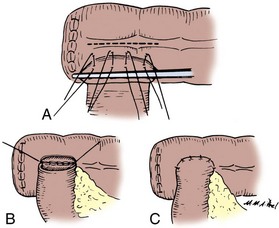
End-to-Side Ileocolic Sutured Anastomosis
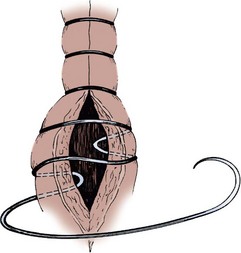
Ileocolonic End-to-End Sutured Anastomosis with Discrepant Bowel Sizes
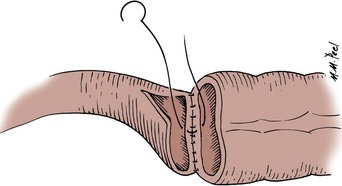
Stapled Anastomoses
Use of Intestinal Segments in Urinary Diversion
1. The left gastric (coronary) artery arises directly from the celiac axis and supplies the lesser curvature.