Arthur I. Sagalowsky, MD, Thomas W. Jarrett, MD, Robert C. Flanigan, MD The etiology, natural history, pathology, detection, and staging of urothelial tumors are presented in Chapters 80, 81, and 82. Only the features that differentiate upper tract tumors from bladder tumors and that are pertinent to treatment are revisited here. Upper urinary tract urothelial tumors involving the renal pelvis or ureter are relatively uncommon, accounting for 5% to 7% of all renal tumors and about 5% of all urothelial tumors (Fraley, 1978; Melamed and Reuter, 1993; Jemal et al, 2004). Because renal pelvic tumors generally are not reported separately, worldwide statistics vary substantially between nations and are not accurate. However, the highest incidence appears to occur in Balkan countries, where urothelial cancers represent 40% of all renal cancers. The frequency of urothelial tumors of the upper tract is increasing, even though the tumors represent only a small percentage of all urothelial neoplasms (McCarron et al, 1982; Richie, 1988; Williams, 1991; Herr, 1998; Messing and Catalona, 1998; Munoz and Ellison, 2000; David et al, 2009). The peak incidence of upper tract tumors is 10 per 100,000 per year, occurring in the age range of 75 to 79 years. Fortunately, synchronous bilateral urothelial upper urinary tract tumors are very rare (Holmang and Johansson, 2004). In one series from Sweden the percentage of upper tract tumors that were bilateral was 1.6%, preceded in 80% of cases by a bladder cancer diagnosis. There may be a decreasing incidence of bilaterality secondary to the prohibition of phenacetin-containing analgesics in the 1960s. An evaluation of data from 1973 to 1996 with use of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database showed 9057 cases, 5379 of the renal pelvis and 3678 of the ureter. In comparison of age-adjusted annual incidence rates, an increase in ureteral neoplasms from 0.69 to 0.73 per 100,000 person-years was found but no change in the incidence of renal pelvic tumors was apparent. In addition, the rate of in-situ neoplasm increased from 7.2% to 23.1%. The incidence of ureteral and renal pelvis tumors in the United States from 1985 to 1994 was 0.73 and 1.0 each per 100,000 person-years, respectively, based on the SEER database (Munoz and Ellison, 2000). The 5-year disease-specific survival was 75% overall and 95%, 88.9%, 62.5%, and 16.5% for in-situ, localized, regional, and distant disease, respectively. Several factors account for this increase in survival for patients with upper tract tumors. Survival of patients with the more prevalent transitional cell carcinoma (TCC) of the bladder is improved owing to more effective diagnosis and treatment. This produces a length-time bias of longer time at risk for development of upper tract tumors. The National Cancer Data Base (NCDB) for the United States for the years 1993 to 2005 identified a total of 334,480 bladder cancers, 15,105 renal pelvis cancers, and 10,128 ureteral cancers (David et al, 2009). There was a significant increase in high-grade tumors in each of the sites during those years. The percentage of early stage tumors increased for both the renal pelvis and the ureter. However, overall, there was no change in survival during those years. Upper tract urothelial tumors are rarely diagnosed at autopsy but rather present clinically during the patient’s lifetime (Ressequie et al, 1978). It also appears that the true incidence of upper tract tumors is increasing as the population ages. Patients with upper tract cancer are generally older than patients with bladder tumors (Melamed and Reuter, 1993). Upper tract tumors rarely present before the age of 40 years, and the mean age at presentation is 65 years (Anderstrom et al, 1989). In conclusion there appears to be a slight increase in the U.S. national incidence of ureteral cancers during the past 2 decades. Fortunately, this has been associated with a slight improvement in the overall and disease-specific survival of patients with upper tract malignant neoplasms. Key Point: Upper Tract Urothelial Tumors Men are about twice as likely to develop upper urinary tract tumors as are women (Greenlee et al, 2000). In addition, whites are about twice as likely as African-Americans to develop upper tract tumors (Greenlee et al, 2000). On the other hand, SEER data suggest that disease-specific annual mortality is greater in black men than in white men (7.4% vs. 4.9%) and greater in women than in men (6.1% vs. 4.4%) (Munoz and Ellison, 2000). As is the case with bladder cancer, women who develop upper urinary tract cancer are 25% more likely than men to die of their disease (Greenlee et al, 2000). Accurate data regarding racial differences in mortality are not available. On the basis of SEER data from the period 1973 to 1996, Rabbani and colleagues (2001) reported that upper urinary tract cancers developed in 657 of 91,245 cases of bladder cancer with adequate follow-up (median 4.1 years). The relative risk for upper urinary tract tumors for white men and women was 64.2% and 75.4% at or before 2 years, 44.3% and 40.5% at 2 to 5 years, 50.8% and 42.1% at 5 to 10 years, and 43.2% and 22.2% at more than 10 years, respectively. These authors concluded that the incidence of upper tract cancers is stable on long-term follow-up and that upper tract surveillance must remain rigorous for an extended period. The incidence of upper tract recurrence has been shown to be higher in patients with carcinoma in situ than in patients with noninvasive papillary TCCs and in patients treated with cystectomy for carcinoma in situ rather than for invasive cancer (Solsona et al, 1997; Premoli et al, 2006; Slaton et al, 1999). Upper urinary tract recurrence is also more likely to occur with high-grade bladder cancer (hazard ratio [HR] 2.16), in T1 versus Ta disease (HR 1.16), and in patients with trigonal or periureteral presentation (HR 1.76) (Wright et al, 2009). On pathologic evaluation, recurrence is most likely to be superficial (Ta, T1, Tis) and to occur in the distal ureter only (47%). However, this finding has not been reported in all series. For example, in patients with Ta, T1, and Tis bladder cancers treated with bacille Calmette-Guérin (BCG), Herr and colleagues (1996) reported a 21% upper tract recurrence rate after a median interval of 7.3 years; the majority of tumors were invasive, and 38.8% of patients with recurrence died of their upper urinary tract disease. Balkan nephropathy is characterized by a degenerative interstitial nephropathy occurring in Balkan countries. Afflicted families display a much higher incidence of upper urinary tract TCC, in some areas 100 to 200 times greater than in nonaffected individuals (Petkovic, 1975). Curiously, bladder cancer incidence is not affected. Tumors are generally of low grade and are more frequently multiple and bilateral than are upper tract TCCs due to other causes (Radovanovic et al, 1985). Balkan nephropathy is familial but not obviously inherited, suggesting an environmental etiology that has yet to be identified. Recent studies suggest that dietary exposure to aristolochic acid may be responsible (Grollman et al, 2007). Interestingly, family members who leave home early in life may not be affected (Radovanovic et al, 1985). Overall, the incidence of this syndrome may be declining (Stefanovic et al, 2008). However, poorer outcomes are still seen in women (HR 2.2), with tumor size larger than 3 cm (HR 2.8), and with stage T3 or T4 disease (HR 3.1) (Dragicevic et al, 2007). Cigarette smoking appears to be the most important of the modifiable risk factors for upper urinary tract cancer, producing an incidence three times that seen in nonsmokers. It appears that this risk is dose related, with a rate as high as 7.2 times normal for long-term (>45 years) smokers (McLaughlin et al, 1992). Former smokers also have a twofold increased risk compared with age-matched persons with no smoking history. This risk declines only partially after smoking ceases. In addition, the risk from smoking seems more often to lead to ureteral rather than to renal pelvic tumors. A relative risk of 1.8 times normal has been described in individuals who consumed more than seven cups of coffee per day (Ross et al, 1989). However, after controlling for cigarette smoking, this risk decreased to 1.3. Analgesic abuse is a well-documented risk factor associated with the development of upper urinary tract cancers (Johansson et al, 1974; Morrison, 1984; McCredie et al, 1986). In one study, 22% of patients with renal pelvic tumors and 11% of patients with ureteral tumors reported a history of analgesic abuse with a latency period of approximately 2 years (Steffens and Nagel, 1988). Renal papillary necrosis and phenacetin consumption also appear to be independent but synergistic risk factors. Each alone resulted in relative risk factors of 6.9 and 3.6, respectively, but together increased risk 20 times (McCredie et al, 1986). Although phenacetin is the most well-described causative agent in analgesic nephropathy, most patients have reported taking combination preparations that included caffeine, codeine, acetaminophen, and aspirin or other salicylates (De Broe and Elseviers, 1998). Histologic findings associated with analgesic abuse include thickening of the basement membrane (pathognomonic) and papillary scarring. Thickening of the basement membrane has been demonstrated in 15% of patients with upper urinary tract tumors and should alert the physician to the presence of analgesic abuse and the subsequent risk of contralateral involvement (Palvio et al, 1987). The degree of papillary scarring also appears to be closely related to tumor grade, although not with the development of squamous metaplasia or squamous cancer (Stewart et al, 1999). Excess inorganic arsenic in drinking water from artesian wells is a major health hazard in certain parts of the world and is associated with an increased risk of upper tract urothelial tumors in addition to other diseases (Tan et al, 2008). Chronic exposure to arsenic in southwestern Taiwan has long been associated with a form of peripheral vascular disease known as blackfoot disease that causes dry gangrene of the extremities. Throughout all regions of Taiwan an increased rate of upper tract urothelial tumors has been noted. These tumors behave in a similar fashion to other upper urinary tract tumors of similar grade and stage. However, there is a distinct female predominance of the upper tract tumors seen in Taiwan in contrast to the male predominance seen in all other areas of the world. Some have postulated that the women may be exposed to arsenic fumes during cooking by steam heat over boiling water. If this is correct it implies an inhaled risk as well as the risk of ingestion from drinking water with high arsenic content. A significantly increased risk for upper urinary tract tumors has been reported for persons employed in chemical, petroleum, and plastic industries (relative risk of 4); patients with exposure to coal or coke (relative risk of 4); and patients with exposure to asphalt or tar (relative risk of 5.5) (Jensen et al, 1988). Aniline dyes, β-naphthylamine, and benzidine have been implicated as causative agents, and tumors can occur at long intervals (up to 15 years or more) after exposure. The development of squamous cell cancer (and less commonly adenocarcinoma) has been shown to be related to chronic bacterial infection associated with urinary stones and obstruction (Godec and Murrah, 1985; Spires et al, 1993). In addition, exposure to cyclophosphamide, an alkylating agent, also appears to confer an increased risk (Brenner and Schellhammer, 1987). Several familial syndromes have been associated with the development of upper tract TCC (Frischer et al, 1985; Orphali et al, 1986; Lynch et al, 1990). Lynch syndrome II, for example, is characterized by the early development of colonic tumors (without polyposis) and extracolonic neoplasms, including upper tract urothelial tumors. Unlike with nonhereditary cancers, these patients are typically younger (mean, 55 years) and are more likely to be female (Lynch et al, 1990). Ureteral tumors occur more commonly in the lower than in the upper ureter. Overall, about 70% of ureteral tumors occur in the distal ureter, 25% in the midureter, and 5% in the proximal ureter (Anderstrom et al, 1989; Messing and Catalona, 1998). This phenomenon may be a reflection of downstream implantation. One area of consensus is that removal of the entire ureter is mandatory when upper urinary tract cancers are removed by nephroureterectomy. Bilateral involvement (either synchronous or metachronous) occurs in 1.6% to 6.0% of sporadic upper tract TCCs (Babaian et al, 1980; Murphy et al, 1981; Kang et al, 2003). Patients with upper urinary tract tumors are at risk for development of bladder cancer, with an estimated incidence that varies in multiple reports from 15% to 75% within 5 years of the development of the upper tract cancer (Kakizoe et al, 1980; Huben et al, 1988; Anderstrom et al, 1989; Hisataki et al, 2000; Miyake et al, 2000; Kang et al, 2003). This high incidence of metachronous bladder involvement suggests that routine bladder surveillance should be performed. Why are upper tract cancers followed by bladder cancers more often than bladder cancers are followed by upper tract cancers? Theories include downstream seeding, longer exposure time to carcinogens in the bladder, and greater number of urothelial cells in the bladder that are subject to random carcinogenic events. Studies have suggested that in high-grade cancers (with associated TP53 gene mutations), which also tend to be more rapidly recurrent, specific gene mutations noted in upper tract cancers are also demonstrated in subsequent bladder cancers (Harris and Neal, 1992; Lunec et al, 1992; Habuchi et al, 1993). In contrast, microsatellite studies in low-grade upper tract cancers, which tend to recur less rapidly in the bladder, have suggested genetic discordance between these upper tract tumors and subsequent bladder cancers in 46% of cases (Takahashi et al, 2000). Upper tract cancers have traditionally been reported to develop in 2% to 4% of patients with bladder cancer, with a mean interval to recurrence of 17 to 170 months (Oldbring et al, 1989; Solsona et al, 1997; Rabbani et al, 2001). Risk factors that have been reported to predict a higher likelihood of the development of upper tract cancers after bladder cancer treatment include stage, grade, multiplicity of tumors in the bladder, presence of ureteral reflux, presence of recurrent carcinoma in situ in the bladder after BCG treatment, multifocal carcinoma in situ in the bladder at the time of cystectomy, and presence of bladder cancers arising close to a ureteral orifice (Zincke et al, 1984; Herr et al, 1992; Hudson and Herr, 1995). Two long-term follow-up series have reported that upper tract recurrence after bladder cancer diagnosis may be much higher than previously thought, occurring in approximately 25% of cases. This phenomenon may be the result of selection of patients, with more high-grade and dysplastic tumors reported in these series (Herr et al, 1996; Solsona et al, 1997). Delayed recurrence is more common in the ureter than in the renal pelvis and appears to occur earlier (at 40 vs. 67 months). In patients treated with BCG for carcinoma in situ of the bladder, upper urinary tract cancer is even more common (about 30% of cases) and appears to occur distally (in the distal, juxtavesical, and intramural portions of the ureter), especially in those patients subjected to cystectomy whose disease is refractory to BCG. Therefore, in cases of high-risk bladder cancer (high-grade T1 disease or carcinoma in situ), imaging of the upper urinary tract should probably be performed annually as part of routine follow-up (Herr et al, 1996). Upper tract urothelial cancers are often associated with a poor prognosis. Up to 19% of patients with upper tract TCC have been reported to present initially with metastatic disease (Akaza et al, 1970). However, one recent study based on a four-institution review suggested that although upper tract urothelial cancers were more often invasive and more often poorly differentiated than bladder cancers, in pathologically matched cohorts recurrence with less aggressive disease, progression to more advanced disease, and death occurred with equal frequency among patients with upper tract and lower tract (bladder) urothelial cancers (Catto et al, 2007). Several studies have suggested that renal pelvic tumors have a better overall prognosis and 5-year disease-specific and recurrence-free survival than do ureteral tumors (Park et al, 2004). However, overall prognosis of upper tract tumors seems to be principally related to tumor stage and to a lesser degree to tumor grade. In one series, 5-year survival was 100% for Ta and Tis, 91.7% for T1, 72.6% for T2, and 40.5% for T3 tumors. Multivariate analysis in this series showed that tumor stage (P = .0001) and age of the patient (P = .042) were the only statistically significant predictors of survival (Hall et al, 1998a). The thin muscle layer of the renal pelvis and ureter probably allows earlier penetration of invasive upper tract tumors through the thinned muscle layer than is seen in bladder cancers (Cummings, 1980; Richie, 1988). In a recent report, 164 patients with upper tract tumors were compared with 2197 patients with bladder cancer. High-grade and deeply invasive TCC occurred in 28.2% of bladder cancers compared with 39.5% of upper tract tumors (Stewart et al, 2005). The renal parenchyma itself may be a barrier to the spread of stage T3 cancers, whereas periureteral tumor extension is a risk factor for early tumor dissemination (Batata and Grabstald, 1976; Guinan et al, 1992a). This anatomic phenomenon may be at least part of the explanation as to why renal pelvic tumors appear to have a better prognosis than ureteral tumors. In one large series of 611 patients treated at 97 hospitals, the 5-year survival rates of patients with stage T3 tumors of the renal pelvis and ureter were 54% and 24%, respectively (Guinan et al, 1992a). TCCs of the upper urinary tract may spread in several different ways, including direct invasion into the renal parenchyma or surrounding structures, lymphatic or vascular invasion, and epithelial spread by seeding or direct extension. It is clear that high-grade tumors demonstrate a greater propensity to invade and that renal parenchymal invasion is the most significant predictor of the development of metastases (95%), followed by vascular invasion (83%) and lymphatic invasion (77%) (Davis et al, 1987). To describe the clonal nature of urothelial tumors of the bladder and upper tract, two theories of the nature of this phenomenon have been proposed. The monoclonality theory describes the multiple tumors as the descendants of a single genetically transformed cell that populates the urothelium. In contrast, the field theory assumes a diffuse “cancerization” that is the result of exposure to a carcinogen and results in the independent development of nonrelated tumors at different sites. Although the majority of the evidence supports the monoclonality theory, most of this evidence has resulted from the study of advanced invasive cancers. It seems that a small but significant proportion of multifocal cancers are, in fact, derived from different clones (Hafner et al, 2002). Epithelial spreading may occur in both antegrade (most common) and retrograde manners. Antegrade seeding is thought to be the most likely explanation for the high incidence of recurrence in patients in whom a ureteral stump is left in situ after nephrectomy and incomplete ureterectomy (Johnson and Babaian, 1979). Lymphatic spread from the upper urinary tract extends to the para-aortic, paracaval, and ipsilateral common iliac and pelvic lymph nodes (Batata and Grabstald, 1976). This extension, of course, depends on the location of the primary tumor and is directly related to the depth of invasion of the primary tumor. Whether lymphadenectomy should be performed routinely and the extent of lymphadenectomy remain controversial (Nakazono and Muraki, 1993; Komatsu et al, 1997). In a series of nephroureterectomy and lymph node dissection, Secin and associates (2005) reported positive lymph nodes in 20% of cases (mean number of lymph nodes sampled, 7.3; range, 1 to 17). Death from disease was 25% for patients with N0 tumors compared with 66.7% in the N1-2 group (P < .001). In this series the presence of suspicious lymph nodes on preoperative imaging studies was the only preoperative predictor of lymph node metastasis on multivariate analysis (P = .02). The most common sites of hematogenous metastases from upper tract tumors are the liver, lung, and bone (Batata et al, 1975). Although it is very rare, direct extension into the renal veins and vena cava may occur in renal pelvic tumors (Jitsukawa et al, 1985; Geiger et al, 1986). The majority of upper tract tumors are urothelial cancers. Of these, the majority are transitional cell in origin; squamous cell cancers and adenocarcinomas represent a small minority (Bennington et al, 1975; Vincente et al, 1995; Flanigan and Kim, 2004). The urothelial lining of the upper urinary tract closely approximates that of the bladder except for the markedly reduced thickness of the muscle layer and the abutting of the urothelium to the renal parenchyma proximally. The epithelial layer is continuous from the level of the calyces to the distal ureter. It has been postulated that the urothelial layer may even “extend” into the collecting ducts, raising the possibility that collecting duct renal cancers may be closely related to urothelial cancers and perhaps better treated by agents used for urothelial cancers (Orsola et al, 2005). This observation needs further confirmation. The walls of the calyces and the pelvis contain fibrous connective tissue and two layers of smooth muscle and are lined on their inner surfaces by transitional epithelium (Dixon and Gosling, 1982) (Figs. 53-1 and 53-2). Thin muscle layers originate in the minor calyces and form a spiral, helical arrangement (Fig. 53–3). The ureter demonstrates two continuous thin muscle layers with a loosely spiraled internal layer and a more tightly spiraled external layer. In the lower third of the ureter, a third outer longitudinal layer is present. All three layers merge with the three layers (inner longitudinal, middle circular, and outer longitudinal) of the bladder wall, which run longitudinally, transversely, and obliquely. Beneath the outer muscle coat is the serosa, made up of loose connective tissue and containing blood vessels and lymphatics (Hanna et al, 1976; Notley, 1978) (Figs. 53-4 and 53-5). (A, © 1999, Rector & Visitors of the University of Virginia.) (© 1999, Rector & Visitors of the University of Virginia.) Several studies have suggested that upper tract urothelial cancers progress through histologic changes from hyperplasia to dysplasia to frank carcinoma in situ in a significant proportion of patients (Heney et al, 1981; McCarron et al, 1982). Carcinoma in situ may be patchy and may extend proximally to the collecting ducts of the kidney (Mahadevia et al, 1983). More severe urothelial dysplastic changes are associated with a greater risk for tumor recurrence in the distal ureter and bladder and a reduced prognosis. Inverted papillomas, although generally considered benign lesions, often have been shown to be associated with either synchronous or metachronous upper tract urothelial tumors (Renfer et al, 1988; Stower et al, 1990; Chan et al, 1996; Cheville et al, 2000). One series demonstrated an 18% incidence of malignancy associated with inverted papilloma of the ureter (Grainger et al, 1990). Other studies have suggested that there are two types of urinary inverted papilloma. The lesions of type 1 behave in a benign fashion, whereas those of type 2 may have a malignant potential. Because there is currently no way to distinguish between these two types, it has been advised that follow-up for all cases of inverted papilloma be continued for at least 2 years after initial diagnosis (Asano et al, 2003). Similarly, these findings suggest close surveillance of the upper tracts for malignancy is warranted when inverted papilloma is diagnosed. TCC makes up more than 90% of upper urinary tract tumors, may present as papillary or sessile lesions, and may be unifocal or multifocal. On histologic examination these lesions are similar to TCCs of the bladder, but the relative thinness of the muscle layer of the renal pelvis and ureter makes invasion through the muscle coat an earlier event. Carcinoma in situ, as in the bladder, can be particularly difficult to identify and can vary in appearance from a whitish plaque to epithelial hyperplasia or a velvety red patch due to increased submucosal vascularity (Melamed and Reuter, 1993). Progression to muscle invasion or invasion into the renal parenchyma or adventitial tissues may occur and is more likely, given the relative thinness of the muscle coat of the upper tracts. Squamous cell cancers make up 0.7% to 7.0% of upper urinary tract cancers (Babaian and Johnson, 1980; Blacker et al, 1985). They are frequently associated with a condition of chronic inflammation or infection or with analgesic abuse (Stewart et al, 1999). These tumors occur six times more frequently in the renal pelvis than in the ureter and are generally moderately to poorly differentiated and more likely to be invasive at the time of presentation. Adenocarcinomas account for less than 1% of all renal pelvic tumors and are typically associated with long-term obstruction, inflammation, or urinary calculi (Stein et al, 1988; Spires et al, 1993). These tumors typically present at advanced stage and display a poor prognosis. A micropapillary variant of TCC in the bladder is associated with aggressive behavior and poor outcome. This histologic subtype is very rare in the upper urinary tract. Holmang and associates (2006) described 26 patients with this entity in the upper urinary tract. Twenty-two patients had stage T3 disease at presentation, and carcinoma in situ or lymphovascular invasion was noted in 64% and 81% of cases, respectively. Five-year survival was only 26.9%, and overall the disease-specific mortality was 77%. Fibroepithelial polyps (benign) (Musselman and Kay, 1986; Blank et al, 1987) and neurofibromas (benign) (Varela-Duran, 1987) are uncommon lesions that are typically treated by simple excision. Multiple types of sarcomas have also been reported to involve the upper urinary tracts, including leiomyosarcomas (Madgar et al, 1988), plasmacytomas (Igel et al, 1991), and angiosarcomas (Coup, 1988). Because of the rare nature of these tumors they are typically treated by excision with adjuvant therapy that is based on the experience with tumors of similar histology occurring elsewhere in the body. Stage is currently the most important predictor of survival in patients with upper tract urothelial tumors (Png et al, 2008). The most commonly used staging system is the TNM system (see later section on staging). Upper tract cancers can spread by direct invasion, mucosal seeding, and hematologic and lymphatic routes. Prognosis decreases as stage increases; the most significant decrease in survival is observed in T3 tumors that have penetrated into the perirenal or periureteral fat (Grabstald et al, 1971). The traditional grading system used for bladder cancer is also applicable to upper urinary tract tumors. Broder’s original system, modified by Ash, grades tumors from grade 1 to grade 4: grade 1 tumors are primarily papillomas, and grade 4 tumors are highly anaplastic and poorly differentiated tumors (Melamed and Reuter, 1993). The World Health Organization’s system, proposed by Mostofi, eliminates papillomas and grades tumors from grade 1 to grade 3. Recently, tumor grading has been divided into low grade and high grade (Epstein, 1998). Papillomas and papillary urothelial neoplasms of low malignant potential are also described. Certainly, tumors of high grade are more likely to invade into the underlying connective tissue, muscle, and surrounding tissues. Tumors of high grade are also more likely to be associated with concomitant carcinoma in situ. There remains disagreement as to whether the location of an upper tract tumor affects prognosis. Several studies have suggested that renal pelvic tumors have a better prognosis than ureteral cancers (Park et al, 2004). In contrast, others have argued that when renal pelvic and ureteral tumors are matched for stage there is no significant difference in prognosis (Hall et al, 1998b). Lymphovascular invasion has been suggested to be an independent prognostic factor for disease-specific survival in upper tract TCC. In a Japanese study, 173 consecutive patients undergoing surgical treatment of upper tract TCC had lymphovascular invasion determined (Kikuchi et al, 2005). Lymphovascular invasion was found in 30% of cases and was more frequent in advanced pathologic stage. Overall 5- and 10-year survival rates were 84.9% and 80.4%, respectively, in the absence of lymphovascular invasion compared with 40.2% and 21.1% with lymphovascular invasion. In three recent single-center and two multicenter series including a total of 1841 patients with upper tract tumors, the prevalence of lymphovascular invasion varied from 23.7% to 37.8%. Lymphovascular invasion correlated with increasing tumor stage and grade and with disease recurrence and disease-specific survival (Akao et al, 2008; Bolenz et al, 2008; Lin et al, 2008; Chung et al, 2009). In multivariate analysis, lymphovascular invasion was an independent and stronger predictor of recurrence and survival than were tumor stage or grade. Kikuchi and colleagues (2009) reported on a large international, 13-center collaborative series of 1453 patients who underwent radical nephroureterectomy for upper tract urothelial tumors. The overall prevalence of lymphovascular invasion was 24%. Lymphovascular invasion correlated with tumor grade stage, lymph node status, and tumor necrosis. In multivariate analysis, lymphovascular invasion was an independent predictor of disease recurrence and survival for patients with either negative lymph nodes or unknown nodal status. However, lymphovascular invasion was not an independent predictor of outcome for patients with positive lymph nodes. Further prospective, large-scale studies are needed to confirm these observations. As with bladder and other solid organ cancers, lymphovascular invasion is the presumed mechanism that leads to regional lymph node and to systemic hematogenous metastases. The molecular and genetic basis of upper tract TCCs appears to be similar to that of TCCs of the bladder. These events have been better described in bladder cancers and are reported in detail in Chapter 80. Briefly, the genetic events leading to the development of upper tract tumors seem to be associated with the overexpression of tumor suppressor genes, including TP53 (on chromosome 17p), the retinoblastoma gene (RB) on chromosome 13q, and several gene foci on chromosome 9 (including the genes for CDKN2C [formerly p18] and CDKN2A [formerly p16] proteins located at 9p21 and 9p32-33). It is generally thought that chromosome 9 abnormalities occur early in the development of these cancers but are not typically associated with high-grade and dysplastic changes, whereas TP53 is more often associated with increased grade and dysplasia. It would also seem, however, that by the time the tumor has been able to invade into the lamina propria both genetic events are likely to have occurred (Spruck et al, 1994). Zigeuner and associates (2004) reported that decreased TP53 immunoreactivity and TP53 overexpression in upper tract tumors were associated with advanced tumor stage and poor prognosis. However, the findings were not independent of stage and grade in multivariate analysis. Eltz and associates (2008) provided a comprehensive review of molecular markers in upper tract urothelial tumors. The TP53 nuclear protein staining of cytology specimens obtained ureteroscopically appears to correlate well with the presence of upper tract TCC. In one study, of 36 TP53-positive specimens 28 had simultaneous evidence of upper tract TCC; 80% of the remaining patients who were evaluated serially also had confirmed TCC. All 14 TP53-negative studies occurred in patients with no sign of concurrent malignant disease on ureteroscopy (Keeley et al, 1997b). Recently the roles of c-MET and RON, members of the MET proto-oncogene family of tyrosine kinases, have been studied in upper urinary tract tumors (Compérat et al, 2008). c-MET overexpression correlated with vascular invasion and a worse clinical outcome, whereas that of RON did not correlate with outcome. Abnormal expression of cyclooxygenase-2 (COX-2) has been reported in many forms of human cancer, including bladder urothelial cancer. Kang and coworkers (2008) reported abnormal expression of COX-2 in stromal cells of upper urinary tract cancers correlated with high tumor stage and grade and poor prognosis. CDKN1B (formerly p27), a cyclin-dependent kinase inhibitor, has also been shown to predict the prognosis of upper tract tumors. In one study, low levels of CDKN1B staining were indicative of a worse disease-specific survival (Kamai et al, 2000). Loss of heterozygosity at 9p21 has been observed in bladder cancer and now in upper tract TCC by microsatellite instability analysis. It has been shown that TCC can occur in patients with hereditary nonpolyposis colorectal cancer (HNPCC) syndrome. Patients with this syndrome show genomic lesions in DNA mismatch repair genes (Amira et al, 2003). Furthermore, an inverted growth pattern of cancer has also been associated with microsatellite instability, with a sensitivity and specificity of 0.82 in one study. This finding suggests that microsatellite instability may serve as a marker for inverted growth in upper urinary tract cancers (Hartmann et al, 2003). Ho and coworkers (2008) have reported that a urine-based assay testing for a total panel of 77 markers for microsatellite instability in 30 patients detected 83.3% of cases of an upper urinary tract tumor. Tumor ploidy has been shown to correlate with survival in upper tract tumors. In one study, tumor aneuploidy was associated with poor 5- and 10-year survival rates of 25% and 0%, respectively (Blute et al, 1988). HIF-1α is a transcription factor that plays an important role in cellular hypoxia adaptation. In a series of patients with upper tract urothelial cancer, positive HIF-1α expression was found in two thirds of cases (was absent in normal urothelium). HIF-1α was significantly associated with high T stage, nodal stage, and grade as well as cancer-specific survival (HR 2.23, P = .004) (Ke et al, 2008). Rapid urine tests for urothelial malignant neoplasms have been studied extensively for the purpose of identifying lower urinary tract tumors. Less is known about their value in upper tract cancers. Urinary levels of NMP22, a nuclear matrix protein–based marker, have been found to be elevated in patients with upper tract cancer (Carpinito et al, 1996). Although the sensitivity of this test for determining the presence of low-grade tumors is probably higher than that of cytology, the specificity is low. Recently, urine fluorescence in-situ hybridization (FISH) was reported to have a sensitivity of 87.5% and a specificity of 80% for detection of upper tract tumors in a small series (Akkad et al, 2007). In one series, an analysis of fibrinogen-fibrin degradation products (AuraTek FDP) was compared with the bladder tumor antigen (BTA) test and urine cytology. In this study, the accuracy of the FDP test was 83% compared with 62% for BTA and 59% for cytology (Siemens et al, 2003). Telomerase activity has been shown to be present in most (>95%) upper tract urothelial cancers. It can be detected in exfoliated urinary specimens in a high percentage of patients and thus may prove to be a potentially useful marker (in addition to conventional cytology) to identify upper tract cancers (Wu et al, 2000). The most common presenting symptom of upper tract urothelial tumors is hematuria, either gross or microscopic. This occurs in 56% to 98% of patients (Murphy et al, 1981; Guinan et al, 1992a; Raabe et al, 1992). Flank pain is the second most common symptom, occurring in 30% of tumors. This pain is typically dull and believed to be secondary to a gradual onset of obstruction and hydronephrotic distention. In some cases, pain can be acute and mimic renal colic, typically ascribed to the passage of clots that acutely obstruct the collecting system. These common symptoms of localized disease (hematuria, dysuria) and of advanced upper tract tumors (weight loss, fatigue, anemia, bone pain) are similar in type and frequency to those of bladder cancer. However, flank pain due to obstruction by tumor or clot is more prevalent in upper tract tumors, having been reported in 10% to 40% of cases (Babaian and Johnson, 1980; McCarron et al, 1983; Richie, 1988; Williams, 1991; Melamed and Reuter, 1993). Flank pain in patients with upper tract tumors does not correlate with either locally advanced tumor stage or worse prognosis, as is the case with bladder cancer. About 15% of patients are asymptomatic at presentation and are diagnosed when an incidental lesion is found on radiologic evaluation. Patients may also present with symptoms of advanced disease, including flank or abdominal mass, weight loss, anorexia, and bone pain. Nearly all upper tract tumors are diagnosed during the patient’s life, and therefore upper tract urothelial cancer represents a rare autopsy finding (Ressequie et al, 1978). Although intravenous pyelography has been the traditional means for diagnosis of upper tract lesions, computed tomographic (CT) urography is increasingly performed today. CT is easier to perform and less labor intensive than intravenous pyelography. It also has a higher degree of accuracy in determining the presence of renal parenchymal lesions. On the other hand, small urinary filling defects (<5 mm) may be missed between the “cuts” of the traditional CT scan. More recently, CT urography has been performed to obtain a three-dimensional image of the upper tracts. This technique appears to be equal to intravenous pyelography in imaging the ureters and renal pelvis (McTavish et al, 2002). With CT urography, the sensitivity for detecting upper tract malignant disease has been reported to approach 100%, with a specificity of 60% and a negative predictive value of 100% (Caoili et al, 2002). CT urography does, however, expose the patient to higher doses of radiation. Radiolucent filling defects, obstruction or incomplete filling of a part of the upper tract, and nonvisualization of the collecting system are the typical findings suggestive of an upper urinary tract tumor. Filling defects, which account for 50% to 75% of cases, typically require the intravenous administration of contrast material to be identified (Murphy et al, 1981; Fein and McClennan, 1986). The differential diagnosis of these defects includes blood clot, stones, overlying bowel gas, external compression, sloughed papilla, and fungus ball. Stones can be ruled out most easily by confirmation of calcification by renal ultrasonography or CT. TCCs have an average density of 46 Hounsfield units (HU) and a range of 10 to 70 HU (Lantz and Hattery, 1984). This is in contrast to an average of 100 HU seen in radiolucent uric acid stones (range, 80 to 250 HU). Thus CT can be useful in distinguishing between these two common causes of radiolucent filling defect on excretory urography or retrograde ureterography. The impact of hydronephrosis and nonvisualization for renal pelvis tumors versus ureteral tumors as indicators of a higher stage is uncertain. Nonvisualization is reported in 20% of renal pelvis tumors, only 33% of which are invasive (McCarron et al, 1983). Nonvisualization is reported in 37% to 45% of ureteral tumors and carried a 60% risk of invasion in one series (McCarron et al, 1983). In other reports there is no correlation of nonvisualization and stage (Batata and Grabstald, 1976; Anderstrom et al, 1989). Hydronephrosis with or without an associated filling defect is linked with invasion in 80% of ureteral tumors (McCarron et al, 1983; Cho et al, 2007). Radiolucent, noncalcified lesions may require additional evaluation by retrograde urography or ureteroscopy, with or without biopsy and cytology. Overall, retrograde urography has an accuracy of 75% in diagnosis of an upper tract malignant neoplasm (Murphy et al, 1981). An incompletely filled or obstructed renal infundibulum or calyx, occurring in 10% to 30% of cases, again typically requires retrograde urography or ureteroscopy to confirm the diagnosis. Obstruction of the urinary tract is a poor prognostic sign for tumor invasion (Babaian and Johnson, 1980). Some have suggested that ultrasonography has sensitivity equal to that of urography in evaluating patients with painless gross hematuria for upper tract malignant disease (Yip et al, 1999; Data et al, 2002). For staging purposes, CT or magnetic resonance imaging (MRI) is most useful in determining the extent of invasion, an associated mass lesion outside the collecting system, and the presence of lymph node or distant metastases (Milestone et al, 1990). CT is also more sensitive than conventional radiography in determining minimally radiopaque substances, making it useful in identifying urine excreted by poorly functioning areas of kidney (as in obstructed areas) (Kenney and Stanley, 1987). The greatest downside of CT or MRI is in the detection of small lesions that may be lost in volume averaging. In one series, CT predicted TNM stage in 60% of patients; it understaged 16% and overstaged 24% (Scolieri et al, 2000). The technical advances achieved in the realm of endoscopic equipment have made the flexible and rigid ureteroscope a key part of the evaluation (and treatment) of upper urinary tract tumors. Diagnostic accuracy can be improved from approximately 75% with excretory or retrograde urography alone to 85% to 90% when it is combined with ureteroscopy (Streem et al, 1986; Blute et al, 1989). Although pyelovenous and pyelolymphatic migration has been reported with ureteroscopy, this phenomenon appears to be uncommon and should not preclude its use (Lim et al, 1993). As with bladder tumors, 55% to 75% of ureteral tumors are low grade and low stage (Cummings, 1980; Richie, 1988; Williams, 1991). Also, like bladder cancers, approximately 85% of renal pelvic tumors are papillary and the remainder sessile. Invasion of the lamina propria or muscle (stage T1 or T2) occurs in 50% of papillary and in more than 80% of sessile tumors. Overall, therefore, 50% to 60% of renal pelvic tumors are invasive into either the lamina propria or muscle. In ureteral tumors, invasion is also more common than in bladder tumors (Anderstrom et al, 1989; Williams, 1991). In addition to visualization of the tumor, ureteroscopy allows more accurate biopsy of suspected areas, with either biopsy forceps or brushing. Good histologic correlation (78% to 92%) between the ureteroscopic biopsy specimen and the final pathologic specimen has been established (Keeley et al, 1997c; Guarnizo et al, 2000). It appears that fresh samples obtained ureteroscopically provide the best chance of predicting eventual pathologic findings. In one study, a cell block from biopsy specimens was prepared when a visible tumor was present, and grades of ureteroscopic biopsy specimens were compared with grades and stages of surgical specimens in 42 cases. Of 30 low- or moderate-grade specimens, 29 (90%) proved to be low- or moderate-grade TCC; 11 of 12 high-grade specimens (92%) proved to be high-grade TCC, and 8 (67%) were invasive (T2 or T3) (Keeley et al, 1997c). In contrast, the urologist’s impression of the tumor grade based on ureteroscopic appearance is likely to be correct in only 70% of cases, suggesting that biopsy is also needed to further define this important aspect of staging (El-Hakim et al, 2004). Because of the small size of ureteroscopic biopsy specimens, a precise correlation with eventual tumor stage is difficult. Therefore, in predicting the tumor stage, a combination of the radiographic studies, the visualized appearance of the tumor, and the tumor grade provides the surgeon with the best estimation of eventual tumor stage. Although, as stated earlier, grading of the tumors may be fairly accurate, staging is much more problematic. Of 40 urothelial tumors staged in one series (40% in the renal pelvis, 20% in the proximal ureter, and 40% in the distal ureter), ureteroscopic grade matched surgical grade in 78% of cases and was less than surgical grade in the remaining 22%. Lamina propria was present in 68% of biopsy specimens (62% of cup biopsies and 100% of loop biopsies), but tumors thought to be Ta were upstaged to T1 to T3 in 45% of cases at the time of complete resection of the lesion (Guarnizo et al, 2000). Therefore, accurate tumor grading on ureteroscopic biopsy is critical in estimating tumor stage. In one series, a biopsy specimen showing grade 3 tumor accurately predicted tumor stage in more than 90% of cases (Skolarikos et al, 2003). Is ureteroscopy (with or without biopsy) necessary in all cases of suspected upper tract tumors? No. In fact, ureteroscopy should probably be reserved for situations in which the diagnosis remains in question after conventional radiographic studies and for those patients in whom the treatment plan may be modified on the basis of the ureteroscopic findings, for example, endoscopic resection. Although there is no evidence that ureteroscopy diminishes the prognosis of a patient destined to proceed to nephroureterectomy, and although the risks of tumor seeding, extravasations, and dissemination are low in experienced hands, these risks are real and should preclude ureteroscopy when it is unnecessary (Hendin et al, 1999). In some cases of upper tract tumors, percutaneous access to the renal pelvis may be required for diagnosis or treatment. In such cases, antegrade urography and uroscopy may be useful for tumor resection, biopsy, or simple visualization. Larger-caliber scopes that can be passed into the renal pelvis in this manner may be particularly helpful in resecting or debulking larger volumes of tumor in this area (Streem et al, 1986; Blute et al, 1989). One must remember, however, that tumor cell implantation in the retroperitoneum and along the nephrostomy tube tract has been reported after these procedures (Tomera et al, 1982; Huang et al, 1995). Urine cytology is a specific tool that is useful in the diagnosis of upper tract carcinomas. On the other hand, the sensitivity of cytology remains an issue. In general, the sensitivity of voided urine (or bladder wash) cytology is directly related to tumor grade. Overall accuracy estimates of the sensitivity of cytology have ranged from about 20% for grade 1 tumors to 45% and 75% for grade 2 and grade 3 tumors, respectively (Murphy and Soloway, 1982; Konety and Getzenberg, 2001). Even if a voided cytology specimen is abnormal in a patient with an upper tract filling defect, one must be cautious in determining the site of origin of the malignant cells. Ureteral catheterization for collection of urine or washings may provide more accurate cytologic results. However, even in this setting, a substantial false-negative or false-positive result (22% to 35%) can be expected (Zincke et al, 1976). It would appear that saline washing provides a better cell yield and improves cytologic results secondary to the release by hydroscopic forces of loosely adherent cells from the urothelium. Still better accuracy can be achieved by brush biopsy through a retrograde catheter or ureteroscope. Sensitivity in the 90% range with specificity approaching 90% may be possible with these techniques (Streem et al, 1986; Blute et al, 1989). Brush biopsies have, however, also been reported to result in severe complications, including massive hemorrhage and perforation of the urinary tract with extravasation (Blute et al, 1981). It appears that the exposure of urothelial cells to ionic, high-osmolar contrast agents as in retrograde pyelography may worsen cytologic abnormalities. Thus, it is probably prudent to obtain cytologic specimens before the use of these agents (Terris, 2004). Early results suggest that FISH also may be useful in the diagnosis of upper tract urothelial tumors. In a study of 21 consecutive upper urinary tract urothelial cancer patients and 10 healthy controls using FISH probes for chromosomes 3, 7, 17, and the CDKN2A (9p21) gene, overall sensitivity of FISH was significantly higher than that of cytology while specificity for both was 100% (Luo et al, 2009). The staging of upper urinary tract tumors parallels the staging of bladder tumors and is presented in Chapter 80. The TNM classification and staging system is the most commonly used. A comparison of the American Joint Committee on Cancer (AJCC) staging system and the TNM system is presented in Table 53–1. Table 53–1 AJCC and TNM Staging and Classification Systems for Upper Urinary Tract Tumors AJCC, American Joint Committee on Cancer. The histologic characteristics and biology of upper tract tumors still affect treatment decisions, technologic improvements notwithstanding. The entity of benign papilloma, which responds favorably regardless of the extent of treatment, is well described in older series of upper tract tumors (Bloom et al, 1970; Batata and Grabstald, 1976). The existence of similar low-grade papillomas of low-grade malignant potential in the bladder remains controversial (Cheng et al, 1999; Cheng and Bostwick, 2000; Oyasu, 2000). It is unclear whether the differences between upper tract papillomas and bladder papillomas are biologic or semantic. Approximately 85% of renal pelvis tumors are papillary; the remainder are sessile. This distribution is similar to that of bladder tumors. However, the stage of upper tract tumors is T1 or T2 in approximately 50% of papillary and 80% of sessile lesions, respectively (Cummings, 1980; Richie, 1988; Williams, 1991). Thus, 50% to 60% of renal pelvis tumors are invasive, in contrast to most bladder tumors, which are noninvasive; 55% to 75% of ureteral tumors are low grade and low stage, but invasion is still more common than among bladder tumors (Anderstrom et al, 1989; Williams, 1991). Patients with upper urinary tract tumors present most often in the sixth or seventh decade of life and thus are generally older than patients with bladder tumors (Melamed and Reuter, 1993). Tumors of the renal pelvis are slightly more common than ureteral tumors (Batata and Grabstald, 1976; Richie, 1988; Maulard-Durdux et al, 1996). Ureteral tumors occur in the distal, middle, and proximal segments in 70%, 25%, and 5% of cases, respectively (Babaian and Johnson, 1980; Anderstrom et al, 1989; Williams, 1991; Messing and Catalona, 1998). After conservative treatment, ipsilateral upper tract tumor recurrence is common in a proximal to distal direction and is seen in 33% to 55% of cases (Mazeman, 1976; Johnson and Babaian, 1979; Babaian and Johnson, 1980; Cummings, 1980; McCarron et al, 1983). Recurrence proximal to the original lesion is rare. Key Point: Upper Tract Tumor Recurrence This high rate of ipsilateral recurrence is due in part to a multifocal field change, which is even more pronounced than in bladder cancer. Areas of atypia, dysplasia, or carcinoma in situ are reported in 60% to 95% of specimens after nephroureterectomy for renal pelvis tumor (Johansson et al, 1976; Kakizoe et al, 1980; Heney et al, 1981; Nocks et al, 1982; McCarron et al, 1983; Melamed and Reuter, 1993). Molecular techniques demonstrate that downward seeding of tumor accounts for some recurrences (Harris and Neal, 1992). Tumor multifocality does not lessen survival of patients independent of stage (Messing and Catalona, 1998). Metachronous bilateral upper tract tumors are rare. In a review of all 768 cases of upper tract tumor reported in western Sweden from 1971 to 1998, the rate of metachronous bilateral tumors was 3.1% and was associated with increased age and short survival time after the event (Holmang and Johansson, 2006). The occurrence of bladder tumors after upper tract tumors, and vice versa, is another expression of the field change, multifocal risk that affects initial treatment decisions. Carcinoma in situ is present in the distal ureter at the time of cystectomy in 7% to 25% of cases (Melamed and Reuter, 1993; Solsona et al, 1997; Herr, 1998); 15% to 50% of all cases of upper tract tumor occur in patients with a history of bladder tumor (Batata and Grabstald, 1976; Babaian and Johnson, 1980). The incidence of upper tract tumor after bladder tumor is 2% to 4% with a mean time to occurrence of 70 months (Shinka et al, 1988; Oldbring et al, 1989; Melamed and Reuter, 1993; Herr et al, 1996). Upper tract tumors are reported in 3% to 9% of patients after cystectomy for bladder cancer in older series (Zincke and Neves, 1984; Mufti et al, 1988). Particular insight into the contemporary risk for upper tract tumor after treatment of bladder cancer is provided in several large series (Solsona et al, 1997; Herr, 1998; Rabbani et al, 2001; Mullerad et al, 2004; Sved et al, 2004; Canales et al, 2006; Tran et al, 2008; Wright et al, 2009). Herr (1998) found that among 307 patients with bladder tumor observed for a median of 12 years there was an overall incidence of upper tract tumors of 23%. The cumulative risks for upper tract tumors were 10%, 26%, and 34% at 5 years, 5 to 10 years, and 15 years, respectively. Upper tract tumors occurred in 26% of another subgroup of 87 patients with bladder tumor observed for more than 15 years. Delayed upper tract tumors were more common in the ureter than in the renal pelvis and appeared at a median follow-up of 56 months. Median time to delayed ureteral versus renal pelvis tumors was 40 months versus 67 months, respectively. Ureteral tumors occurred in 29% of 66 patients who were successfully treated with intravesical BCG for carcinoma in situ. In a subset of 105 patients who underwent cystectomy for BCG-refractory carcinoma in situ, ureteral carcinoma in situ was present in the distal, juxtavesical, and intramural portions of the ureter in 35%, 68%, and 81% of cases, respectively. In a retrospective study from the same center, treatment outcome in patients with upper tract tumor was worse in patients with a prior history of bladder cancer (superficial or invasive) independent of upper tumor stage (Mullerad et al, 2004). Similarly, Sved and colleagues (2004) reported upper tract tumors in 2% of patients (5 of 235) observed for a mean of 42 months after radical cystectomy for bladder cancer. Upper tract tumor was diagnosed at a mean follow-up of 39.6 months, because of hematuria in four cases, and on routine intravenous urography in the remaining case. Presence of tumor in the prostatic urethra of the cystectomy specimen was the only initial tumor feature that was associated with a higher risk of subsequent upper tract tumor. This may be a predictor of a higher risk of multifocal tumor in such cases. Outcome was poor with disease-related mortality in four of the cases. In contrast, in a review of 91,245 patients with bladder cancer from the SEER database for 1973 to 1996, upper tract tumor occurred in 657 patients (0.7%) at a median follow-up of 4.1 years (Rabbani et al, 2001). These patients had lower tumor stage and improved disease-specific survival than did patients with primary upper tract tumors. Canales and coworkers (2006) found that patients with two or more stage Ta bladder cancer recurrences within 12 months were at increased risk for upper tract tumors and that surveillance of the upper tracts is indicated. In an update on the earlier study by Herr and associates (1996), Tran and associates (2008) found that the risk for upper tract recurrence after cystectomy for bladder cancer was 4% and 7% at 3 years and 5 years, respectively. Patients who had any tumor in the ureter at the time of cystectomy, including only carcinoma in situ, were at greatest risk for upper tract recurrence. Wright and colleagues (2009) reviewed 99,338 patients with bladder cancer in the SEER registry from 1988 to 2003. Upper tract tumors occurred in 0.8% of patients, at a median time of 33 months. Seventy-one percent of these were seen within 5 years, and only 6% of the total occurred after 10 years. On multivariate analysis high-grade and non–muscle-invasive bladder tumors correlated with a higher risk for subsequent upper tract tumors. The latter point likely relates to a lower mortality rate from the original bladder cancer and thus a greater competing risk for upper tract disease. Solsona and colleagues (1997) reported that carcinoma in situ was present in the distal ureter in 25% of 138 cystectomies performed for bladder carcinoma in situ, compared with only 2.3% and 2.9% among 786 and 179 cases of stage Ta to T1 versus stage T2 disease, respectively. This may indicate that bladder carcinoma in situ carries a higher risk of multifocal disease than do other forms of bladder tumor. The incidence of bladder tumor after treatment of upper tract tumor is 20% to 75% (Batata and Grabstald, 1976; Kakizoe et al, 1980; Nocks et al, 1982; Huben et al, 1988; Anderstrom et al, 1989; Sakamoto et al, 1991; Williams, 1991; Melamed and Reuter, 1993; Hisataki et al, 2000; Kang et al, 2003; Matsui et al, 2005). Bladder tumor after upper tract tumor occurs earlier than the reverse, at a median of 21 months versus 86 months afterward, respectively. Both bladder tumor and contralateral upper tract tumor after initial unilateral upper tract tumor are examples of the multifocal nature of urothelial tumor. In one study, subsequent bladder tumors appeared earlier than did contralateral upper tract tumors (Kang et al, 2003). Renal insufficiency was associated with a higher risk of contralateral upper tract tumor. The occurrence of bladder tumor after treatment of upper tract tumor may be due to a field effect, distal tumor seeding, or both. Association of a higher bladder tumor incidence after upper tract tumor multifocality supports a role of distal seeding (Matsui et al, 2005). However, reports of distinct tumor clones by microsatellite analysis support a field effect (Takahashi et al, 2001). The paradoxical finding that the risk of subsequent bladder tumor is inversely related to upper tract tumor size and stage may reflect a higher and earlier risk of death from the primary tumor in these cases. In the reports by Hisataki and coworkers (2000), Matsui and associates (2005), and Terakawa and colleagues (2008), increased upper tract tumor stage at the time of nephroureterectomy correlated with a higher risk for subsequent bladder tumor. In a recent European multicenter study reported by Novara and coworkers (2008) prior bladder tumor before upper tract tumor was the only independent risk factor for bladder tumor after nephroureterectomy in multivariant analysis. Raman and associates (2007) reported that the grade, but not the stage, of the prior upper tract tumors correlated with the pathologic findings of the subsequent bladder tumors. Three particular forms of upper tract urothelial tumors, those seen in Balkan nephropathy, those associated with analgesic abuse, as well as those seen in arsenic-endemic regions of Taiwan, each have an even higher tendency to multiple and bilateral recurrences than do sporadic tumors (Markovic, 1972; Petkovic, 1975; Mahoney et al, 1977; Johansson and Wahlquist, 1979; Melamed and Reuter, 1993; Stewart et al, 1999; Tan et al, 2008). The typically low-grade nature of the tumors and the frequent renal insufficiency seen in Balkan nephropathy underscore the importance of conservative treatment when possible. The degree of scarring of renal papillae seen in phenacetin abuse correlates in a dose-dependent manner with the risk of high tumor grade and progression. Calcification of renal papillae after analgesic abuse is associated with development of squamous carcinoma of the renal pelvis (Stewart et al, 1999). There is a strong correlation of grade and stage for upper tract tumors. The single most important determinant of outcome is tumor stage (Tables 53-2 and 53-3) (Bloom et al, 1970; Grabstald et al, 1971; Batata et al, 1975; Wagle et al, 1975; Babaian and Johnson, 1980; Cummings, 1980; McCarron et al, 1983; Huben et al, 1988; Anderstrom et al, 1989; Guinan et al, 1992b; Terrell et al, 1995; Messing and Catalona, 1998). Table 53–2 Correlation of Tumor Stage and Grade for Upper Tract Urothelial Tumors Data from McCarron JP Jr, Mills C, Vaughn ED Jr. Tumors of the renal pelvis and ureter: current concepts and management. Semin Urol 1983;1:75–81. Table 53–3 Literature Review of Overall Survival of Patients with Upper Tract Urothelial Tumors (Renal Pelvis or Ureter) by Stage and Grade Key Points: Staging of Upper Tract Tumors Upper tract tumors spread in the same ways as bladder tumors do, through lymphatic and hematogenous routes and by direct extension into contiguous structures. Thus, the common metastatic sites are the lungs, liver, bones, and regional lymph nodes. Preoperative evaluation for the extent of disease includes chest radiography, abdominal CT, liver function tests, and occasional bone scintigraphy. The thin muscle layer of the renal pelvis and ureter may allow earlier penetration of invasive upper tract tumors than is seen in bladder neoplasms (Cummings, 1980; Richie, 1988). However, this is not a valid reason for radical versus conservative treatment if a lesion is either noninvasive or invasive and organ confined (stage T2) (Gittes, 1980). The renal parenchyma may be a barrier, slowing distant spread of stage T3 renal pelvis tumors. In contrast, periureteral tumor extension carries a high risk of early tumor dissemination along the periureteral vascular and lymphatic supply. Improved survival of patients with stage T3 renal pelvis tumors versus ureteral tumors has been reported by several investigators (Batata and Grabstald, 1976; Guinan et al, 1992a; Park et al, 2004). Guinan and colleagues confirmed this observation among 611 patients treated at 97 hospitals and in a collection of 250 cases reported in the literature (Guinan et al, 1992a). The 5-year survival rates for patients with stage T3 tumors of the renal pelvis and ureter were 54% and 24%, respectively. In a multivariate analysis, patients with ureteral tumors had a higher local and distant failure rate than did those with renal pelvis tumors of the same stage and grade (Park et al, 2004). Renal pelvis and upper ureteral tumors spread initially to para-aortic and paracaval nodes, whereas distal ureteral tumors spread to pelvic nodes (Batata et al, 1975; Heney et al, 1981; Nocks et al, 1982; Mahadevia et al, 1983; McCarron et al, 1983;Jitsukawa et al, 1985; Geiger et al, 1986). Open conservative surgery may be applied in selected cases when nephron sparing for preservation of renal function is required (Gittes, 1966, 1980; Petkovic, 1972a, 1972b; Mazeman, 1976; Johnson and Babaian, 1979; Babaian and Johnson, 1980; Cummings, 1980; Wallace et al, 1981; Tomera et al, 1982; McCarron et al, 1983; Zincke and Neves, 1984; Bazeed et al, 1986; Ziegelbaum et al, 1987; Messing and Catalona, 1998). Tumor in a solitary kidney, synchronous bilateral tumors, and predisposition to form multiple recurrences, as in endemic Balkan nephropathy, are all reasons to consider nephron sparing (Fig. 53–6). Pyeloscopy as an initial diagnostic component to open conservative surgery has been supplanted by retrograde or percutaneous antegrade renal endoscopy (Huffman et al, 1985). Direct endoscopic visualization of the lesion and biopsy with cup forceps or brush establish a definitive diagnosis and tumor grade (Gill et al, 1973). The brush biopsy has renewed value because tissue obtained by small cup biopsy forceps used through narrow-caliber flexible endoscopes is limited. Preoperative determination of the stage of renal pelvis tumors remains difficult. Large size, broad base, and nonpapillary pattern favor tumor invasiveness. These improvements in initial diagnosis and assessment of tumor stage and grade allow more specific treatment, including conservative surgery when it is indicated.
Epidemiology and Etiology
Incidence and Mortality Rates
Variations by Sex and Race
Upper Urinary Tract Tumors after Known Bladder Cancer
Etiology and Risk Factors
Balkan Nephropathy
Smoking
Coffee Consumption
Analgesics
Arsenic
Occupation
Chronic Inflammation, Infection, or Exposure to Chemotherapeutic Agents
Heredity
Natural History
Location and Distribution of Tumors
Progression to Muscle Invasion and Metastases
Patterns of Spread
Epithelial
Lymphatic
Hematogenous
Pathology
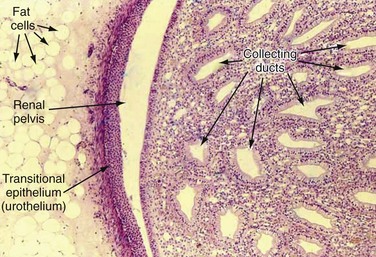
Normal Upper Tract Urothelium
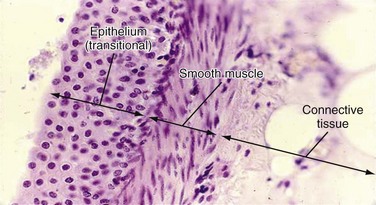
Renal Pelvis and Calyces
Ureter
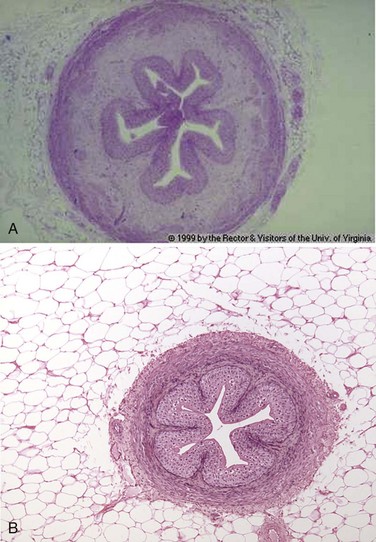
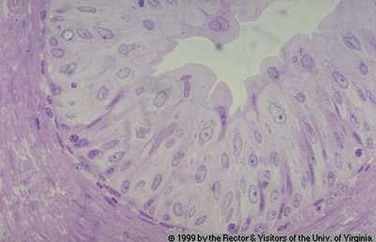
Abnormal Urothelium
Metaplasia and Dysplasia
Benign Lesions: Inverted Papillomas and von Brunn Nests
Transitional Cell Carcinoma
Non–Transitional Cell Carcinoma
Squamous Cell Cancers
Adenocarcinomas
Micropapillary Variant
Other Miscellaneous Tumors
Prognostic Factors
Stage
Grade
Location
Lymphovascular Invasion
Molecular Biology (Chromosome Abnormalities) and Markers
TP53
c-MET
COX-2
CDKN1B
Loss of Heterozygosity
Ploidy-Flow Cytometry
Overexpression of Hypoxia-Inducible Factor-1α (HIF-1α)
Other Markers
Diagnosis
Symptoms and Signs
Radiologic Evaluation
Ureteroscopic Evaluation and Biopsy
Antegrade Endoscopy
Role of Cytology and Other Tumor Markers
Staging
TNM Staging System
Primary Tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Ta
Papillary noninvasive carcinoma
Tis
Carcinoma in situ
T1
Tumor invades subepithelial connective tissue.
T2
Tumor invades the muscularis.
T3
Tumor invades periureteral fat (for renal pelvis only).
Tumor invades beyond muscularis into perinephric fat or the renal parenchyma.
T4
Tumor invades adjacent organ or through the kidney into the perinephric fat.
Lymph Nodes (N)
NX
Regional lymph nodes cannot be assessed.
N0
No regional lymph node metastases
N1
Metastasis to a single lymph node, 2 cm or less in greatest dimension
N2
Metastasis in a single lymph node, more than 2 cm but not more than 5 cm in greatest dimension; or multiple lymph nodes, none more than 5 cm in greatest dimension
N3
Metastasis in a lymph node, more than 5 cm in greatest dimension
Distant Metastasis (M)
MX
Distant metastasis cannot be assessed
M0
No distant metastasis
M1
Distant metastasis
AJCC Staging System
TNM Classification System
0
T0
I
Ta, Tis, T1, N0, M0
II
T2, N0, M0
III
T3, N0, M0
IV
T4 or any T, N+, M+
LOCATION AND STAGE
HIGH GRADE (%)
Pelvis
Low
5
High
91
Ureter
Low
26
High
64
5-YEAR SURVIVAL (%)
Tumor Grade
1-2
40-87
3-4
0-33
TNM Stage
Ta, T1, Tcis
60-90
T2
43-75
T3
16-33
T4
0-5
N+
0-4
M+
0
Treatment
Open Nephron-Sparing Surgery for Renal Pelvis Tumors: Pyelotomy and Tumor Ablation and Partial Nephrectomy
Indications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Urothelial Tumors of the Upper Urinary Tract and Ureter