Serologies
Cytomegalovirus
Herpes simplex virus 1 and 2
Hepatitis A, B, and C viruses
Epstein–Barr virus (EBV)
Varicella zoster virus
Human immunodeficiency virus
Rapid plasma regain
Tuberculin skin test or QuantiFERON-TB Gold or T-SPOT.TB test
Endemic fungi
Non-serologic tests
Urinalysis with culture
Pap-smear with pelvic exam
Prostate exam
Dental evaluation
Chest radiograph
Tests to consider after thorough history
Toxoplasma serology
Human T-cell leukemia virus
Colonoscopy
Ultrasound of the gallbladder
Stool culture, test for ova and parasite
In addition to these screening tests, a vaccination history is obtained for tetanus, diphtheria, pertussis, polio, influenza, pneumococcus, hepatitis A and B, and Haemophilus influenzae type B, measles, mumps, and rubella [5, 6]. The different immunizations that should be questioned for in the initial evaluation of a potential transplant recipient and antibody titers quantified are listed in Table 27.2. Testing titers should be done prior to transplantation because some vaccinations, namely, the live-attenuated viral vaccines including oral polio (no longer available in the United States), nasal influenza and varicella zoster virus (VZV), measles, mumps, rubella, and bacillus Calmette–Guerin (BCG) are contraindicated after transplantation [7]. These vaccines should be given several months prior to transplantation. Varicella vaccine can be given to children safely in pediatric pre-transplant patients and should be given to adults who have low titers prior to transplant [8].
Table 27.2
Immunizations
Name | Immunization type | Re-immunization | Contraindications |
|---|---|---|---|
Influenza | Inactivated, component (IM) | Yearly | Intranasal formulation as this is live-attenuated virus |
Pneumococcal | Component | Second dose 5 years after initial | _ |
Tetanus-diphtheria | Toxoid | Every 10 years, booster at 5 years if deep puncture wound | Neurological reaction or anaphylaxis after previous dose |
Varicella | Live attenuated | Second dose after 1–2 months | Immunosuppressed; only given prior to transplantation; not given to those who have received blood products in the previous 6 months |
Meningococcal | Component | 3–5 years if antibody titers decline | _ |
Haemophilus Influenza type B | Conjugated | Second dose after 2 months | _ |
Hepatitis A | Formalin-inactivated | Booster at 6–12 months (Havrix), booster 6 months (Vaqta) | _ |
Hepatitis B | Recombinant | Repeat 1 and 2 months. Check titer 1 month after last shot and if <10, give booster | _ |
Measles, Mumps, Rubella | Live attenuated | Second dose 4–6 years after | Anaphylaxis to first dose of vaccine, pregnancy, immunosuppressed |
Kidney transplant donors also undergo several tests prior to donation. Donors may harbor infectious diseases that can be transmitted to the recipient through the donated organ. There have been reports of viral transmission of adenovirus, West Nile, parvovirus, hepatitis B and C, herpes simplex, HIV, human T-lymphotropic virus, influenza, parainfluenza, rabies, polyomaviruses, and lymphocytic choriomeningitis virus through the transplanted organ [9]. In addition, bacterial, mycobacterial, fungal, and parasitic infections have also been transmitted from a donor to recipient. These include Enterococcus, Acinetobacter, Ehrlichia, E. coli, Klebsiella, Legionella, Listeria, Lyme disease, Nocardia, Serratia, Staphylococcus aureus, Streptococcus, Rocky Mountain spotted fever, Syphilis, Candida, Coccidioides, Cryptococcus, histoplasmosis, Aspergillus, Tuberculosis, Trypanosoma cruzi, Schistosomiasis, Strongyloides, malaria, and Balamuthia [9]. Despite the various numbers of possible transmitted infections from organ donor to recipient, transmission is reported in <1 % of donors [9].
Current recommendations for pre-transplant testing in living donors include tests for CMV, HIV 1 and 2, hepatitis B surface antigen and surface antibody, hepatitis B core antibody, HCV antibody and nucleic acid testing (NAT), rapid plasma regain (RPR) testing, human T-lymphotropic virus (HTLV), tuberculosis testing, and Epstein–Barr virus (EBV) [10]. Screening urinalyses with cultures are performed on living donors. For deceased donors, blood cultures are typically obtained to rule out a systemic bacterial infection. Systemic infections are generally a contraindication to accepting a deceased donor organ. Prior to organ procurement, deceased donors are given antibiotics, usually a cephalosporin. At the time of organ transplantation, a recipient will also receive prophylactic antibiotics, with or without placement of antibiotics intravesically during the transplant surgery [11, 12]. The various tests that potential kidney donors undergo prior to donation are listed in Table 27.3.
Table 27.3
Donor evaluation and testing
Serologies |
Cytomegalovirus |
EBV |
Human immunodeficiency virus 1 and 2 |
Hepatitis B virus surface antigen and surface antibody |
Hepatitis B virus core antibody |
Hepatitis C virus antibody |
Hepatitis C and HIV nucleic acid testing |
Rapid plasma reagin |
Human T-lymphotropic virus |
Tuberculin skin test |
Non-serologic tests |
Urinalysis with culture |
Blood and sputum culture (if deceased donor) |
Pregnancy test for premenopausal donors |
PSA for men >50 years |
Consider ANA if family history of lupus |
If recipient of the donor has polycystic kidney disease, obtain US kidneys if >30 years old, CT if less than 30 years old |
Posttransplantation Infections
Timing of Posttransplant Infections
Historically, posttransplant infections have been described according to the length of time from transplant: 1 month, second through 6 months, and greater than 6 months. A timeline with various possible infectious organisms that can affect post-kidney transplant recipients are shown in Fig. 27.1. The differences in infection types seen during these periods are due to the decreasing amounts of immunosuppressants needed after transplant and the different exposures that patients experience after their transplant. There are instances, however, where patients will have significant increases in their net immunosuppression, such as during episodes of acute and/or chronic rejection which require pulse steroids or increases in their overall immunosuppressant doses. The infectious risk again increases during this time period, similar to the early transplant period when patients’ immunosuppression doses were higher. The timeline is not rigid as patients receive antibiotic and antiviral medications that may change the time at which infections occur in the absence of prophylaxis or treatment.
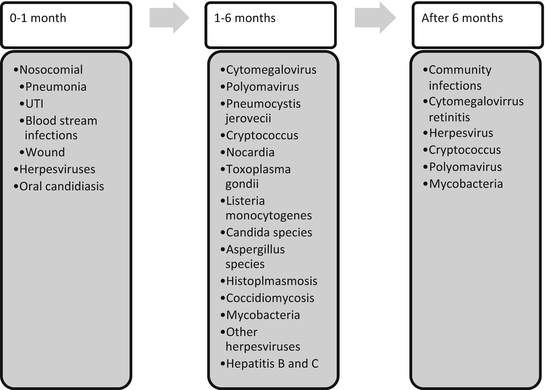

Fig. 27.1
Timeline and infectious organisms after kidney transplant
In the first month posttransplant, most infections are nosocomial and related to the surgical procedure. Relatively few are due to occult infections in the transplant recipient or transmitted from the donor. Common infections occurring within the first month of transplant include surgical wound infections, UTIs, pneumonia, and catheter-related blood stream infections from indwelling central venous catheters. Methods for decreasing infectious risk include meticulous observation of sterile conditions during surgery and removal of foreign bodies such as central lines, urinary catheters, ureteral stents, drainage tubes, and endotracheal tubes as soon as feasibly possible. Early ambulation and pulmonary care can help reduce the risk of pneumonia. Isolation of the patient and use of gowns, masks, and gloves for health-care personnel or visitors, even if the patient is neutropenic, has been shown to be unnecessary [13]. Several viral infections may reactivate in the first month, including herpes simplex virus (HSV) 1 and 2, human herpes virus (HHV)-6, EBV, CMV, and VZV in the absence of prophylaxis.
By the second to sixth month after transplantation, the nosocomial infections have historically been replaced by infections from opportunistic and reactivated organisms due to the relatively high doses of immunosuppressants that patients receive in the early posttransplant period. These infections included CMV, Pneumocystis jirovecii, Cryptococcus neoformans, Nocardia species, Toxoplasma gondii, Listeria monocytogenes, and Candida and Aspergillus species. With steroid avoidance and minimization, these opportunistic infections, except for CMV, are not commonly seen. However, EBV, CMV (in the absence of adequate prophylaxis), herpes viruses such as HSV 1 and 2, HHV-6, HHV-7, HBV, HCV, polyomavirus, Mycobacteria, histoplasmosis, and coccidiomycosis can reactivate during months 2–6 or later.
By 6 months posttransplantation, most centers have decreased immunosuppression to lower maintenance doses, and the antilymphocyte antibody induction effects have diminished. Consequently, patients will then usually suffer from similar infections affecting the general population including UTI’s, influenza, diarrhea, cold sores, and pneumonia. A few notable exceptions to this include viral infections, including CMV retinitis that can occur late and herpes or zoster lesions which may be sentinel lesions for CMV. Several infections paired with prophylaxis medications that can help prevent disease are listed in Table 27.4.
Table 27.4
Prophylaxis use in transplant recipients
Infectious agent | Prophylaxis |
|---|---|
Wound infections | Perioperative antibiotics |
Urinary tract infections | Trimethoprim–sulfamethoxazole, ciprofloxacin |
Herpes simplex viruses | Acyclovir |
Varicella zoster virus | Acyclovir, immune globulin |
EBV | Acyclovir, valganciclovir, ganciclovir |
Cytomegalovirus | Ganciclovir, valganciclovir |
Pneumocystis jiroveci | Trimethoprim–sulfamethoxazole |
Oral candidiasis | Nystatin swish and swallow, clotrimazole troche |
Bacterial Infections
Notable posttransplant bacterial infections include those that affect immune-competent patients and those that do not usually affect non-immunosuppressed patients. Bacterial infections commonly seen during the immediate posttransplant surgical period include those related to the surgery. These include infections of the blood stream due to central lines, lungs, surgical site, and urinary tract.
UTIs commonly occur in transplant patients who do not receive antibiotic prophylaxis, with an incidence of 29–79% [14, 15]. Symptoms for UTI include dysuria, frequency, and urgency. Some patients may not experience symptoms, and the only evidence of infection would be through routine urinalysis and culture. Risk factors for UTIs include female gender, recurrent UTI’s pre-transplant, prolonged duration of dialysis after surgery, diabetes, neurogenic bladder, prolonged use of bladder catheters postsurgery, allograft trauma, polycystic kidney disease, and ureteral anastomotic complications [14, 16, 17]. Empiric treatment for UTIs should cover the most common organisms but should be tailored to the organism once culture and specificities return. Common pathogens leading to UTIs include bacteria in the Enterobacteriaceae family (Escherichia coli, Proteus, Citrobacter, Enterobacter, and Klebsiella), Pseudomonas aeruginosa, Enterococci, and Staphylococci [18]. Due to the high rates of urinary infections, vigilance in implementing preventative measures is important. It has been shown that the early removal of a urinary catheter decreases the incidence of UTIs [19]. Many centers prescribe antibiotic prophylaxis for their posttransplant patients. One randomized study showed the beneficial effects of prophylactic use of double strength trimethoprim–sulfamethoxazole (TMP–SMX) 320 mg/1,600 mg) [18]. In this study, patients had no difference in the rate of UTIs with a catheter in place; however, after catheter removal the overall risk of bacterial UTI was <10 % [18]. Though there was not an increased rate of resistant bacterial infections in the treated group, patients who had bacterial infections were more likely caused by resistant bacteria [18]. An additional benefit in using TMP–SMX as antibiotic prophylaxis for UTIs is its effectiveness as prophylaxis for Pneumocystis jirovecii (formally known as Pneumocystis carinii).
Patients may also have persistent bacteriuria posttransplant, which should be evaluated further with ultrasound or computed tomography (CT) for a nidus of infection such as an abscess. Early detection and treatment of UTIs is important, as these infections can lead to more serious infections, including bacteremia and pyelonephritis. Pyelonephritis with bacteremia will present with fevers, pain or tenderness over the allograft, and usually allograft dysfunction. The urinary tract is the most common source for bacteremia in transplant patients, with a reported 2-week mortality of 11 % [20].
Pneumonia
Pneumonias are also a common cause of infections and hospitalization in post-kidney transplant patients. Prior to transplant, the incidence of pneumonias in these patients is 37 episodes per 100 patient-years, then sharply increases in the first month post-kidney transplant to 216 episodes per 100 patient-years, then declines to approximately 60 episodes per 100 patient-years by the end of the first year, and remains steady thereafter [21]. Overall, patients are 3.62 times more likely to have pneumonia in the first 12 months after transplant than after 12 months [22]. Cough, fever, shortness of breath, evidence of oxygen desaturation, and pulmonary physical findings are important signs and symptoms in the initial evaluation. Diagnostic tests include blood and sputum gram stain and culture, chest X-ray and computed tomography, and bronchoscopy or open lung biopsy with cultures are done. Despite testing, however, the etiology of the pneumonia is not found in a significant proportion of patients, between 23 and 31 % from two studies [22, 23]. Organisms found on testing in one study include bacteria (mostly S. aureus, S. pneumonia, P. aeruginosa, K. pneumonia, Acinetobacter, Haemophilus, Enterobacter, E. coli, and Nocardia), Tuberculosis, mixed bacteria, fungus, Pneumocystis, atypical organisms, CMV, and other viruses [22, 23].
Due to the relatively high mortality rates (12.5–40 % in some studies) associated with pneumonia, broad-spectrum antibiotics usually in the form of a third-generation cephalosporin, a β-lactam with β-lactamase inhibitor, or a quinolone are initiated [24, 25]. Some will add vancomycin depending on the clinical situation and local methicillin-resistant Staphylococcus aureus (MRSA) resistance rates. Consideration should be made for adding an antifungal if there is no improvement. Once the etiologic factor has been found, therapy can be directed and simplified to the specific organism.
Wound Infections
The incidence of wound infections after kidney transplantation has decreased from 25 to 56% in earlier studies to 4.8% in a more recent study [26, 27]. Risks for wound infections include increased body mass index, urine leak, need for reoperation through the primary incision, immunosuppressants (particularly use of Mycophenolate Mofetil), and diabetes [26]. Improvements in the incidence of wound infections in kidney transplantation are due to the use of prophylactic antibiotics and bladder irrigation with antibiotic solution.
Nocardiosis
Nocardia species is a ubiquitous organism in the environment usually inhaled or directly inoculated through soil particles [28]. The most common species found are N. asteroides, N. farcinica, and N. brasiliensis. Disease presentation varies in the immunocompromised host. Most infections caused by Nocardia are primarily pulmonary, manifesting with fever, chills, cough, and dyspnea. Nocardia will also commonly disseminate to other organ systems in nearly half of affected patients, including central nervous and integumentary system involvement [28]. Patients with central nervous system (CNS) involvement may present with confusion, fevers, headache, vision changes, weakness, and lethargy from brain abscesses. Skin findings include subcutaneous nodules, abscesses, swelling, and cellulitis. Diagnostic methods for Nocardiosis include skin, sputum or bronchoalveolar lavage (BAL) culture, X-ray or computed tomography of the lungs, and MRI of the brain [29]. Treatment for Nocardiosis usually includes TMP–SMX for a prolonged course. Other treatments include third-generation cephalosporins, aminoglycosides, minocycline, and amoxicillin-clavulanate [28–30].
Legionella
Legionella usually presents as pneumonia with a peripheral patchy infiltrate or consolidation on CXR. Legionella pneumophila is the most common Legionella species causing infection and is associated with epidemics from contaminated respiratory equipment and contaminated heating and cooling systems and drinking water. Diagnostic testing for this condition includes a urine antigen for Legionella, which identifies serogroup 1, and culture. Other tests used for diagnosis are the direct fluorescent antibody (DFA) test in BAL or sputum specimens [31]. The treatment of choice for Legionnaires’ disease is azithromycin, fluoroquinolones, followed by doxycycline and TMP–SMX. Rifampin is not used for monotherapy but is used in combination therapy. Azithromycin has no interaction with calcineurin inhibitors (CNIs), while rifampin increases the cytochrome P-450 activity, leading to significantly lower CNI levels and adjustment in dosing while on this medication.
Listeriosis
Listeriosis is a disease caused by Listeria monocytogenes, an organism that can contaminate meats, vegetables, and dairy products through soil and water contact and enter the gastrointestinal tract to cause disease. Most infections will occur early in the posttransplant period, and usually in the summer months, with one study showing that some transplant patients’ fecal matter can contain Listeria [32]. Patients may present with gastroenteritis, CNS infection, and meningitis, with up to a 33% mortality [33]. Diagnosis of CNS disease requires a lumbar puncture, which reveals polymorphonuclear monocytes and low glucose. There may or may not be Gram-positive bacilli on smear/culture and should be repeated if no bacteria are seen on the initial test. Ampicillin is the first-line treatment for this infection, followed by TMP–SMX.
Viral Infections
Herpes Viral Infections
The herpes family includes eight herpes viruses including HSV 1 and 2, VZV, EBV, CMV, and HHV-6, 7, and 8. Each can cause significant reactivation in patients taking immunosuppressants. The different HHVs and the disease associated with each virus are listed in Table 27.5.
Table 27.5
Human Herpes viruses and resultant diseases
HHV-1 Herpes simplex 1 (HSV 1) | Herpes labialis |
|---|---|
HHV-2 Herpes simplex 2 (HSV 2) | Herpes genitalis |
HHV-3 Varicella zoster virus (VZV) | Chicken pox |
Shingles | |
HHV-4 EBV | Mononucleosis |
Burkitt’s lymphoma | |
Posttransplant lymphoproliferative disorder (PTLD) | |
Nasopharyngeal carcinoma | |
Oral hairy leukoplakia | |
HHV-5 Cytomegalovirus (CMV) | Cytomegalovirus viremia, syndrome, disease |
Salivary gland virus disease | |
HHV-6 Human herpes virus 6 | Roseola subitum |
HHV-7 Human herpes virus 7 | Roseola subitum |
Pityriasis rosea | |
HHV-8 Human herpes virus 8 | Kaposi’s sarcoma |
Castleman’s disease | |
Effusion lymphoma |
Cytomegalovirus
CMV is the most common herpes virus affecting post-kidney transplant recipients with significant morbidity. CMV may be detected in urine, blood, or saliva in 50–75 % of renal transplant recipients after transplant, but only 8–32% of recipients will experience symptoms related to CMV disease [34]. Factors affecting the development of clinical disease include the serostatus of both the donor and recipient at the time of transplant, with the highest risk of developing symptomatic disease in D+/R− patients, and less risk in D−/R+, D+/R+ D−/R− Other factors affecting the development of CMV symptoms and disease include induction with antilymphocyte antibody, other active viral infections, and the strength and amount of maintenance immunosuppressants [35]. Allograft rejection treatment can increase the rate of CMV infection, while CMV infection and the required reduction in maintenance immunosuppression as part of treatment for CMV can predispose patients to allograft rejection and chronic allograft dysfunction [34, 36]. New infections can occur in seronegative recipients from their seropositive donor or from an exposure, while previously seropositive recipients can have a new infection from their donor or from reactivation of latent disease in the recipient.
Symptoms and signs of CMV disease usually appear as fever, malaise, diarrhea, thrombocytopenia, neutropenia, and transaminitis. Recipients can also present with symptoms related to involvement of the pulmonary, hepatic, gastrointestinal, nervous, retinal, renal, and cardiac systems [34, 36]. CMV is also associated with acceleration of coronary artery disease. Diagnosis of CMV viremia requires evaluation of the peripheral blood for viral load using several possible methods such as pp65 antigenemia assay, amplification of CMV DNA or RNA, shell vial, and hybridization [36]. The nucleic acid-based tests are preferred for accuracy and quantification. In some cases, tissue invasive disease may not have a detectable viral level, making a tissue biopsy necessary for diagnosis.
Due to significant morbidity and mortality related to CMV infection, several different strategies have emerged: general prophylaxis, selective prophylaxis, preemptive, and deferred methods of management [37]. Selective prophylaxis entails providing prophylaxis specifically to recipients who are D+/R− and at highest risk of developing symptomatic disease. General prophylaxis provides antiviral prophylaxis to all recipients regardless of CMV serostatus. Preemptive therapy refers to monitoring CMV viral load, and treating viremia prior to the development of symptoms. The deferred approach to CMV viremia is where patients with viremia undergo treatment only when they manifest symptoms. These strategies differ significantly, with no uniformly agreed upon approach.
Prophylactic therapy is effective in decreasing CMV disease, infection, and mortality [38]. Medications used primarily for CMV prophylaxis are ganciclovir and valganciclovir. Although acyclovir, valacyclovir, and CMV immune globulin have been used in some centers, these have been shown to have limited efficacy [38–40].
There are a few issues that arise from following the prophylactic approach. Ganciclovir-resistant CMV develops in 7% of recipients who receive this antiviral, largely due to the viral kinase UL97 gene responsible for phosphorylating ganciclovir and making it an active drug [41]. Second, prophylaxis is expensive [42]. Third, the use of prophylaxis may only lead to later onset of CMV disease after the completion of the prophylactic antivirals [43, 44]. Of note, there is no consensus on the number of months of prophylactic therapy for patients after transplantation. Some studies advocate approximately 3 months with close monitoring for the development of CMV viremia while others advocate a longer course of 6 months due to the emergence of late onset CMV disease and the cost-effectiveness of a longer course compared to a shorter course [43, 45–48].
Doses for prophylaxis are oral ganciclovir 1,000 mg three times daily or valganciclovir 900 mg daily. One study showed no significant differences between using either drug, and valganciclovir has better bioavailability compared to oral ganciclovir (70% vs. 7%) [49, 50]. Of note, both drugs require dose adjustment for those with renal insufficiency. The different CMV risk categories, prophylaxis medications, and medications used for treatment of CMV are listed in Table 27.6.
Table 27.6
CMV risk, prophylaxis, and treatment
CMV risk categories |
High risk |
CMV D+/R− |
Intermediate risk |
CMV D+/R+ or CMV D−/R+ |
Low risk |
CMV D−R− |
Prophylaxis medications |
Ganciclovir 1,000 mg oral three times daily Valganciclovir 900 mg oral daily |
Treatment medications |
Ganciclovir intravenous 5 mg/kg every 12 h |
Valganciclovir 900 mg oral twice daily |
Cidofovir |
Foscarnet |
Leflunomide |
The preemptive approach may be associated with decreased development of late onset CMV disease, but the cost of weekly or bimonthly viral load assessments may not be more cost-effective compared to prophylaxis [37, 51]. The deferred strategy of treating CMV disease when symptoms develop usually requires intravenous ganciclovir 5 mg/kg every 12 h or oral valganciclovir 900 mg twice a day for 2–3 weeks or until symptoms abate. One study has shown no difference in outcomes between the oral valganciclovir and IV ganciclovir in the treatment of CMV disease [52]. In addition to antiviral treatment, reduction in mycophenolate and azathioprine may be required. Cidofovir and foscarnet can also be used to treat symptomatic CMV disease, but their use is limited by nephrotoxicity. If treated inadequately, CMV may develop resistance to ganciclovir and other medications used to treat the disease. Two prominent genes, UL97 and UL54, encoding the viral protein kinase and DNA polymerase respectively, confer resistance to ganciclovir (UL97/UL54) and foscarnet and cidofovir (UL54) [53]. Leflunomide and maribavir may prove useful in treating patients with ganciclovir-resistant CMV strains [54].
Herpes Simplex Virus
HSV 1 and 2 are associated with herpes labialis and genitalis, respectively. Initially patients will have acute infections followed by a period of dormancy within the sensory nerve ganglia. In the first several months posttransplant, patients may experience reactivation of their latent disease. This manifests as ulcerations of the mucus membranes or herpetiform skin lesions. Others can present with more serious complications such as esophagitis, hepatitis, encephalitis, pneumonitis, and disseminated disease. Diagnosis is achieved by lab tests including the Tzanck smear and culture. Polymerase chain reaction (PCR) testing in the cerebral spinal fluid (CSF) is preferred for the evaluation of possible CNS disease. Treatment for simple oral or genital lesions is acyclovir 200 mg orally 4–5 times daily. Other antiviral medications include valacyclovir and famciclovir. Serious infections require intravenous acyclovir dosed at 5–15 mg/kg every 8 h. The resistance of HSV to acyclovir is 4–7 % in immunocompromised hosts [55].
Varicella Zoster Virus
Reactivation of VZV is a concern in renal transplant recipients, as over 90% have been exposed to the virus prior to transplant. The diagnosis is made with the discovery of pain, shingles, or vesicles in a dermatomal distribution with confirmation through Tzanck smear, DFA, or PCR. Like HSV, VZV can present with hepatitis, encephalitis, pneumonia, and disseminated disease. Treatment for a zoster outbreak is acyclovir 800 mg orally five times daily. Alternatives are famciclovir and valacyclovir. Those with disseminated disease should be treated with intravenous acyclovir 10 mg/kg every 8 h for 7 days. In addition to this, patients should be placed in negative pressure rooms until all lesions have dried to prevent the spread to individuals who have never been exposed to VZV. If exposed, these seronegative individuals should receive varicella immune globulin within 72 h and oral acyclovir 200 mg five times daily. Up to 25% of patients with zoster will have post-herpetic neuralgia, which can be treated using tricyclic antidepressants, narcotics, gabapentin, pregabalin, and tramadol [56].
Epstein–Barr virus
EBV is a virus which most adults have been exposed to previously, with a 95% seropositive prevalence among adults. Kidney transplant recipients who are seronegative or seropositive collectively have a 20% rate of new infection or reactivation within the first year posttransplant [57]. Diagnostic testing includes plasma PCR testing for viral DNA to detect new or reactive disease. EBV can cause symptoms of fatigue, mononucleosis-like syndrome, Burkitt’s lymphoma, nasopharyngeal carcinoma and, in posttransplant patients, posttransplantation lymphoproliferative disease (PTLD), which is potentially fatal. The risks of developing PTLD include EBV seronegative status, immunosuppressants, and youth. The long-term incidence of PTLD is 1–2 % in kidney transplant recipients [58]. In addition, the use of one of the newer immunosuppressants, belatacept, is associated with a higher risk of PTLD than conventional immunosuppression including antilymphocyte therapy [59].
The majority of PTLD is due to B-cell proliferation of affected EBV cells that normally remain dormant under T-cell control but proliferate after T-cell suppression with immunosuppressants. Prophylaxis used to prevent CMV viremia can also be used to prevent EBV viremia. Some centers have employed a monitoring strategy to detect viremia early in seronegative recipients and have treated these patients with rituximab to help prevent the development of PTLD [60]. Studies have also shown that administration of EBV-specific cytotoxic T-lymphocytes to seronegative recipients can be used as prophylaxis for EBV [61, 62].
Human Herpes Virus 6, 7, 8
HHV-6 is a beta herpes virus that causes roseola infantum, or exanthema subitum in children. Most people have had the infection prior to adulthood, and transplant recipients can experience reactivation of their disease, with similar symptoms to CMV. Other potential complications of reactivation are hepatitis, encephalitis, and hemophagocytic syndrome [63]. Antiviral treatment for HHV-6 is similar to that used for CMV, although it is controversial whether HHV-6 is susceptible to ganciclovir [64]. HHV-7 is another HHV that many have been previously exposed and is associated with roseola and pityriasis rosea in children. Reactivation of this infection can also be associated with CMV disease [65]. Unlike HHV-6, ganciclovir is clearly inadequate to treat HHV-7, and affected transplant recipients may require cidofovir [66]. HHV-8 is the etiologic virus of Kaposi’s sarcoma (KS), Castleman’s disease, and primary effusion lymphoma [67]. Less than 3% of the population are seropositive for HHV-8, with pockets of higher prevalence >25 % in parts of Italy, Greece, and Africa [67]. Though the virus is sexually transmitted, HHV-8 can be acquired from the donated kidney [68]. The incidence of KS among transplant recipients is 0.1–5 % and tends to present within the first 3 years after transplantation in an aggressive manner [69]. Treatment usually involves decreasing immunosuppression, radiation, and chemotherapy, although some cases may regress with the use of rapamycin [70].
Prophylaxis used for CMV may also decrease the incidence of KS in transplant recipients indirectly by preventing CMV reactivation [71].
Hepatitis Viruses
The prevalence of chronic hepatitis B surface antigen positive (HBVsAg+) carriers is between 10 and 25% [72]. The presence of chronic liver disease in renal transplant recipients is associated with an increased risk of sepsis and liver failure [73]. The viruses affect the immune response by suppressing the immune system, which has a twofold effect of decreasing the likelihood of allograft rejection but also increasing the risk for infection [74].
Hepatitis B (HBV) is a DNA virus that can cause significant liver disease in kidney transplant recipients, including hepatitis and cirrhosis. Disease can result from worsening of previously controlled disease, new infection, or transmission through the donor allograft. The possibility of worsening or reactivated disease in those who are HBV+ underscores the need to obtain a baseline liver biopsy prior to renal transplantation. Concern for reactivation or seroconversion arises from data that recipients who are HBsAg− and HBsAb+ (hepatitis B core antibody positive (HBcAb+) have progressed to reactivation of their disease and disease progression [75]. In a meta-analysis performed to evaluate studies of lamivudine for treatment of HBV, the overall estimate for clearance of HBV using lamivudine was 91%; however, the estimate for development of lamivudine resistance was 18% [72]. Adefovir, tenofovir, entecavir, and emtricitabine are other antivirals that are useful to treat HBV. Entecavir is less nephrotoxic than tenofovir.
HCV is a single-stranded RNA virus with a prevalence of 10–41 % among transplant recipients [76]. Transmission of the virus is hematologic, thus making HCV transmissible through renal allografts from donors with HCV, though there is rare transmission if the donor is anti-HCV antibody positive and HCV RNA negative. In renal allograft recipients with HCV, chronic hepatitis develops in more than 60% [77]. Both recipient and allograft survivals decrease in HCV-positive recipients (65 and 49%) compared to HCV negative recipients (80 and 63%) [78]. Unfortunately, recipients with HCV can develop cirrhosis, hepatocellular carcinoma, membranous glomerulonephritis, membranoproliferative glomerulonephritis, cryoglobulinemia, and thrombotic microangiopathy. As a result of the complexities of HCV infection long term in the kidney transplant recipient, the pre-transplant evaluation is extensive, including liver biopsy, HCV genotyping, and RNA quantification.
Treatment using pegylated-interferon alpha, ribavirin, and amantadine can be offered prior to transplantation, as treatment with interferon can lead to acute rejection. Over the last decade, there have been strides in the treatment of hepatitis C, particularly in patients who are infected with genotype 1. Two new direct-acting antivirals in a class of protease inhibitors have been developed and tested for the treatment of hepatitis C. Their mechanism of action is to inhibit the action of NS3/NS4A HCV serine protease, effectively preventing the formation of important nonstructural proteins of HCV [79]. The first is telaprevir which has been studied in a population of patients with hepatitis C genotype 1 who had not previously been treated. In phase I and II trials, the use of this oral drug for 12 weeks with peg-interferon and ribavirin for 24 weeks led to the sustained viral response (SVR) of 69% [80, 81]. Genotype 1b responds better to telaprevir than genotype 1a [82]. In phase III studies of previously treated or treatment naïve HCV genotype I infected individuals, investigators found significant SVR in those treated with telaprevir (70–80 % SVR) compared to those who received standard therapy with pegylated-interferon alfa-2b and ribavirin (20–40 % SVR) [83, 84]. The medication has side effects, making it difficult to tolerate, including pruritus, anemia, and rash.
Another serine protease inhibitor that has proven effective in clinical trials is boceprevir. In phase III studies, in patients previously treated for HCV genotype I, the rate of SVR was 59–66 % in those patients treated with boceprevir compared to those who received standard therapy with pegylated-interferon alpha 2a and ribavirin 21% [85]. Because of the previously found difference in response rates between blacks and whites, investigators sought to differentiate the SVR in a non-black cohort and black cohort of patients using boceprevir [86, 87]. For the non-black cohort, the SVR was 67–68 % in those receiving boceprevir compared to those who received standard therapy (40%) [87]. For the black cohort, the use of boceprevir led to a 42–53 % SVR compared to those treated with pegylated-interferon alpha 2a and ribavirin (23 %) [87]. Boceprevir was associated with anemia requiring erythropoietin-stimulating agents during therapy.
There are concerns for the use of triple therapy for HCV genotype 1 treatment in the posttransplant population. Most concerning is the interaction between the protease inhibitors and CNIs [88]. Both telaprevir and boceprevir inhibit cytochrome P450 3A4, which is involved in the metabolism of CNIs. It has been shown in healthy volunteers that the trough levels of cyclosporine and tacrolimus increase by 4.6-fold and 70-fold, respectively, in the presence of steady-state telaprevir [89]. They also found that the half-life of both cyclosporine and tacrolimus increased significantly with telaprevir [89]. Use of the protease inhibitor drugs requires close monitoring in the presence of CNI use. These serine protease inhibitors have not yet been approved in transplanted patients, though several studies are currently ongoing in cohorts of liver transplant recipients to determine their efficacy and safety in this population of patients.
Influenza
Influenza viral infections affect posttransplantation recipients similar to those who are not immunosuppressed, manifesting with fevers, myalgias, headache, and cough. However, more serious presentations may occur in immunosuppressed patients, including primary viral and secondary bacterial pneumonias, rhabdomyolysis, and encephalitis. Due to the potential seriousness of the disease, transplant recipients and their close contacts should receive the influenza vaccine yearly. At the time of the H1N1 pandemic of 2009, transplant recipients safely received the injectable version of the regular and H1N1 vaccine, with better immune response when the two were given simultaneously [90, 91]. Recipients should not, however, receive the live-attenuated version of the vaccine, as this could lead to active disease. Anyone with an egg allergy or not able to receive the vaccine should receive alternative medications for prophylaxis, if exposed or for treatment. These include the M2 blockers amantadine and rimantadine and the viral neuraminidase inhibitors, zanamivir and oseltamivir. The M2 blockers are only effective against influenza A, while the neuraminidase inhibitors have activity against both influenza A and B. Influenza A can develop resistance to the M2 blockers, and the antiviral resistance of the circulating influenza strain should be taken into consideration. Adjustments for renal function must be made as these drugs are renally cleared.
Risk of Allosensitization with Vaccination
There has been some discussion over the risk of allosensitization after immunizations. It has previously been shown that anti-HLA antibodies are found at very low levels in healthy males after influenza vaccination, likely as a result of natural antibody production to cross-reactive epitopes from infectious organisms or perhaps through vaccinations [92, 93]. Anti-HLA antibodies have been discovered after non-allogeneic stimulus in healthy adults after immunization with hepatitis B vaccine by 2 months postvaccination in 9/20 patients [94]. There are mixed results from investigators who have evaluated allosensitization after influenza vaccinations. Two studies found that a certain small number of patients (5.6–17.3 %) develop new de novo anti-HLA antibodies directed against both donor and non-donor HLA after receiving the influenza vaccine [95, 96]. One criticism to the study by Katerinis is that the influenza vaccine was an adjunctive vaccine which could potentiate the development of anti-HLA antibodies [97]. Another study found augmented cellular immunity after influenza vaccination, but not in the humoral response in transplanted patients, while the non-transplanted individuals experienced an enhanced cellular immune response, with a few developing new alloantibodies [98]. There are several studies that did not observe development of HLA antibodies in stable posttransplant patients after administration of influenza vaccine [90, 99–101]. Overall, it is still considered safe to administer the influenza vaccine to transplanted patients 3 months posttransplantation, with a very small likelihood of development of anti-donor-specific antibodies. The risks of the development of donor-specific antibodies must be weighed against the benefit of vaccination and prevention of an influenza infection in transplant recipients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






