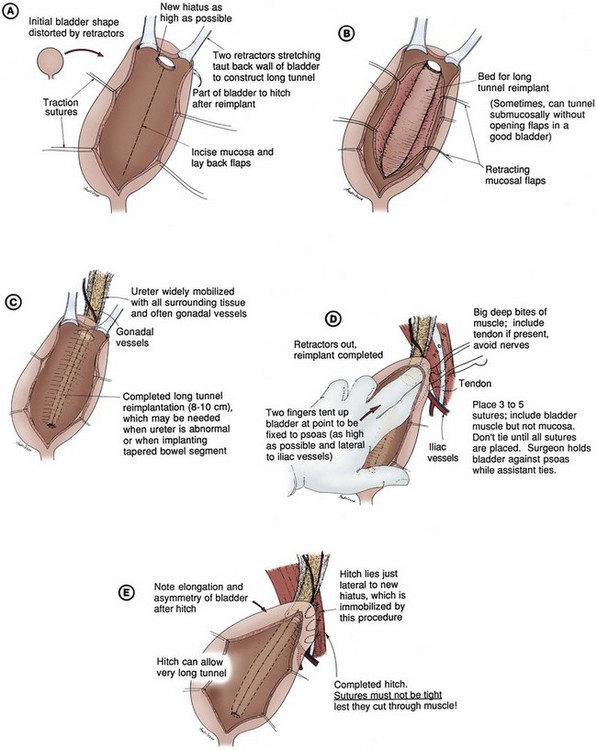
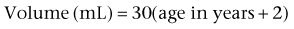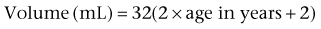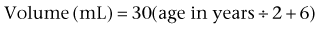Mark C. Adams, MD, FAAP, David B. Joseph, MD, FACS, FAAP Complex patients with bladder dysfunction may have bladder neck and external sphincteric problems as well. This chapter covers techniques to increase native outflow resistance in the pediatric population. Certain techniques, such as Young-Dees-Leadbetter bladder neck repair, are presented elsewhere in this section of this text. Others (i.e., sling, collagen injection, and artificial urinary sphincter) have been used more extensively in adults; again, in that setting, the focus in this chapter is on adaptations of those procedures for children. Perhaps the most important contribution affecting lower urinary tract reconstruction was the introduction of clean intermittent catheterization (CIC) by Lapides and colleagues (1972, 1976). Because many pediatric patients with bladder and sphincteric dysfunction will not void adequately after reconstruction, a reliable means for easy catheterization without discomfort is an important part of their care. The work of Mitrofanoff (1980) stimulated interest in continent abdominal wall stomas within pediatric urology, and several effective techniques have since been developed. That experience serves as a nice lead into a discussion of continent urinary diversion in children because construction of an effective efferent limb that provides continence and a reliable means for catheterization is often the most challenging aspect of such diversion. “Pure” continent urinary reservoirs, such as Indiana and Kock pouches, are occasionally performed in children, and that experience is reviewed. More frequently in children, some of the patient’s native urinary tract is used in the reconstruction. This is done to maintain as much urothelium-lined tissue in the urinary tract as possible, to minimize the amount of needed bowel, and, potentially at least, to minimize the morbidity to the patient. The result then is a spectrum of repairs between bladder augmentation and continent urinary diversion but rarely a continent urinary reservoir in the classic sense. Several decades ago, many such reconstructions were performed after previous, permanent urinary diversion with use of bowel (Hendren, 1998). Today, however, few children are initially treated with permanent diversion. Most reconstructive procedures are now undertaken primarily to correct a problem in the native urinary tract (hydronephrosis, infection, incontinence) unresponsive to medical management or after temporary diversion. Children with bladder and sphincteric dysfunction are among the most complex seen in pediatric urology; among others, patients with diagnoses such as exstrophy, persistent cloaca and urogenital sinus, posterior urethral valves, bilateral single ectopic ureters, and prune-belly syndrome may be involved. For most pediatric urologists, patients with myelomeningocele make up the majority of patients requiring this type of surgical intervention. Consequently, many of the results discussed herein focus on the neurogenic population. Appropriate urinary storage requires a reservoir that is compliant and of age-appropriate capacity. Age-based capacity may be estimated by formulas proposed by Koff (1983): or by Kaefer and associates (1997c) for children younger than 2 years: and for children older than 2 years: Compliance is defined as the change in bladder volume divided by the change in pressure. Normally, the bladder is considered a highly compliant vesicle in that it will accommodate an increasing volume of urine without a corresponding increase in intravesical pressure. Multiple factors contribute to this property. Initially, the bladder is in a collapsed state that allows the storage of urine at low pressure by simple unfolding. As it expands, detrusor properties of elasticity and viscoelasticity take effect. Elasticity allows the detrusor muscle to stretch without an increase in tension until it reaches a critical volume. This volume should be greater than the expected bladder capacity. The viscoelastic bladder property allows a subtle continuous pressure change that occurs with bladder filling. It is associated with a small rise in pressure as the bladder stretches, balanced by a corresponding rapid pressure decay (Zinner et al, 1976; Wagg and Fry, 1999). With slow natural bladder filling there should be no net change in bladder pressure until capacity is reached. This viscoelastic bladder property is also defined as stress relaxation and can be overcome when the rate of bladder filling exceeds normal parameters, resulting in a pattern consistent with poor compliance (Mundy, 1984; Finkbeiner, 1999). This artifact of testing is often noted when urodynamic assessment is performed at an excessive filling rate for the child’s age or size (Bauer and Joseph,1990; Joseph, 1992). These properties of elasticity and viscoelasticity will eventually be overcome in every child, and at that point the bladder pressure rapidly rises. Favorable dynamics for urine storage include a thin bladder wall with an appropriate composition of muscle and collagen allowing expression of normal elastic and viscoelastic properties. Factors adversely affecting normal compliance include detrusor hypertrophy, fibrosis, outlet obstruction, and recurrent urinary tract infections (Mundy, 1984; Joseph, 1994). Continence during urinary storage requires a closed bladder neck at times supported by a contracted external urinary sphincter. Fixed obstruction, neurogenic dysfunction, and chronic inflammation can affect any or all of these passive parameters, resulting in resting bladder hostility and clinical manifestations of poor compliance, upper tract deterioration, and incontinence (Brading, 1997). Under normal conditions the active phase of voiding requires the bladder to contract after descent of the bladder neck (Morrison, 1997). Reflexive opening of the bladder neck and sequential relaxation of the external urinary sphincter allow low-pressure balanced voiding and complete elimination of urine. Again, obstruction, neurogenic dysfunction, and chronic infection can cause physiologic changes preventing coordinated function of the detrusor, bladder neck, and external sphincter, defined as dyssynergy (Mundy et al, 1985). A poorly functioning external sphincter from denervation fibrosis may also prevent appropriate relaxation, causing elevated voiding pressure against a fixed outlet. Finally, detrusor pathophysiologic changes may prevent a sustained, coordinated bladder contraction and full elimination of all urine. Much like obstruction, vesicoureteral reflux in the presence of a bladder abnormality may be primary or secondary. Differentiation between the two can be difficult. On occasion the history is helpful. If reflux was not present initially in a patient with neurogenic dysfunction but developed later, it is typically secondary to bladder hostility. Most reflux in patients with neurogenic dysfunction requiring bladder augmentation is likely to be secondary; reflux in patients with other problems (exstrophy, prune-belly syndrome, or posterior urethral valves) may be either primary or a fixed secondary problem if it persists until the time that bladder reconstruction is necessary. Previous work has suggested that reflux truly secondary to bladder dysfunction may not need surgical correction if the bladder is adequately managed. In those reports, neurogenic bladder patients requiring augmentation did not undergo reimplantation for secondary reflux, and virtually all of the low-grade reflux resolved with augmentation alone (Nasrallah and Aliabadi, 1991; Morioka et al, 1998; Lopez Pereira et al, 2001; Soylet et al, 2004; Juhasz et al, 2008). It is interesting to speculate whether reflux is even a significant problem if a large, compliant bladder is achieved (Soylet et al, 2004). Bacteria may ascend without reflux after reconstruction (Gonzalez and Reinberg, 1987). Experience with certain forms of continent diversion has not shown an increased risk of pyelonephritis in the absence of any antireflux mechanism if the reservoir is adequate (Helal et al, 1993; Pantuck et al, 2000). Although the authors agree that most low-grade secondary reflux is likely to resolve with adequate treatment of the bladder alone, our preference is to correct reflux at the time of bladder reconstruction, unless it is low grade or clearly secondary, because the morbidity is low. After bladder reconstruction, many patients have chronic bacteriuria, particularly those who catheterize to empty, and the absence of reflux, at least theoretically, may decrease the likelihood of ascent to the kidney. Caution must be taken when the treatment of chronically dilated and scarred ureters is considered. Correction of reflux in that setting is certainly appropriate, but one must be careful not to trade reflux for more problematic obstruction. Overaggressive tapering or tunneling of such ureters may be fraught with complications. Bladder dysfunction is a composite of physiologic abnormalities, and it is helpful to assess each component of passive and active bladder function independently. Elevated passive filling pressure becomes clinically pathogenic when a pressure higher than 40 cm H2O is chronically reached (McGuire et al, 1981; Wang et al, 1988; Weston et al, 1989). Pressures at this level sustained for a time impair ureteral drainage, which may result in pyelocalyceal changes, hydroureteronephrosis, and decreased glomerular filtration rate. In addition, persistent elevation in filling pressure can result in acquired vesicoureteral reflux (Sidi et al, 1986a; Cohen et al, 1990). Pharmacologic management can play a role in decreasing filling pressure, particularly when hyperreflexic detrusor contractions are present. A combination of medications and intermittent catheterization has a positive impact, particularly in children with neurogenic dysfunction (Rink and Mitchell, 1984; Aslan et al, 2002; Verpoorten et al, 2008). Bladders that are poorly compliant because of irradiation or chronic inflammatory processes are not as likely to respond in a positive fashion to this form of therapy. When compliance is unaffected by medical management, augmentation cystoplasty will be required to improve the storage characteristics. It is expected that detrusor contractions resulting in effective emptying will be significantly diminished after augmentation. With rare exceptions, intermittent catheterization should be taught to and accepted by the patient and caretaker before urinary reconstruction is contemplated. One of the most important contributions in the care of children with bladder dysfunction came with the acceptance of CIC described by Lapides and colleagues (1972, 1976) based on the work of Guttmann and Frankel (1966). The effective use of CIC has allowed the application of augmentation and lower tract reconstruction to groups of patients who had not previously been candidates. The principle of intermittent catheterization allows the reconstructive surgeon to aggressively correct storage problems by providing an adequate reservoir and good outflow resistance. Good spontaneous voiding, although a goal, is not imperative because catheterization can be used for emptying. Intermittent catheterization can maintain a physiologic state of complete emptying on a regular basis. When incontinence occurs during the filling phase because of poor outlet resistance, it is essential to evaluate not only the bladder neck and external urinary sphincter but also the detrusor characteristics. Clinical experience has shown that once appropriate resistance is achieved at the bladder neck through operative intervention, adverse detrusor characteristics may become unmasked and result in high-pressure urinary storage or uninhibited contractions not previously documented (Bauer et al, 1986; Churchill et al, 1987; Dave et al, 2008b). For that reason, provocative urodynamic assessment with occlusion of the bladder neck is important before any bladder neck reconstruction in an attempt to identify children who will be at risk. Normal synergistic voiding occurs when the bladder neck descends, relaxes, and opens followed by relaxation of the external urinary sphincter and subsequent detrusor contraction. The cascade results in low-pressure voiding. Dysfunctional voiding during this active bladder phase occurs from discoordinated activity of the bladder neck, external urinary sphincter, and detrusor and can result in urinary incontinence. In addition, when neurogenic dysfunction leads to detrusor-sphincter dyssynergy, high-pressure voiding results during a period of time, having a negative impact on the bladder and upper urinary tract (Mundy et al, 1982; Bauer et al, 1984). A similar clinical situation occurs with a fixed, fibrotic external sphincter. Initial treatment usually involves pharmacologic management and mechanical elimination of urine from the bladder through CIC in an attempt to bypass the abnormal voiding mechanics. Renal function should be assessed in any patient undergoing bladder reconstruction, particularly if hydronephrosis or severe renal scarring is present. Demos (1962) and Koch and McDougal (1985) have demonstrated that urinary solutes, particularly chloride, are absorbed from urine in contact with the mucosa of small and large bowel. For patients with normal renal function, the kidneys are able to handle the reabsorbed load of chloride and acid without obvious difficulty. Patients with decreased renal function, however, may develop significant metabolic acidosis secondary to such reabsorption. If acidosis exists preoperatively, it will invariably worsen if urine is stored in small or large intestinal segments (Mitchell and Pizer, 1987). The first component of renal function to deteriorate after obstruction or infection is typically concentrating ability. Patients with compromised function may generate enormous volumes of urine. The bladder volume achieved through bladder reconstruction must accommodate the patient’s urinary output for an acceptable time, usually about 4 hours. Patients with renal failure or other medical problems may conversely develop oliguria. Low urinary output may affect an augmented bladder or bowel reservoir because there is greater potential for collection and inspissation of mucus. There is also less urine for dilution and buffering of gastric secretions if stomach is used. Abnormal function of other organ systems also influences the risk of bladder reconstruction with use of intestinal segments. Reabsorption of ammonia by large or small intestinal segments in contact with urine may be dangerous for patients with hepatic failure (McDougal, 1992a). Some medications excreted in urine may be reabsorbed by bowel mucosa (Savauagen and Dixey, 1969). Therefore, liver function tests and arterial blood gas studies may be appropriate for some patients. Careful history should be taken of the patient’s preoperative bowel function; this is particularly true of adult patients who may have acquired, or secondary, gastrointestinal problems. Obviously, short-gut syndrome is a concern among patients with cloacal exstrophy, prior bowel resections, or a history of significant irradiation. A history of chronic diarrhea or fecal incontinence preoperatively should signal concern about use of the ileocecal valve in urinary reconstruction. Urodynamic assessment of the lower urinary tract plays an essential role in considering bladder reconstruction. It can provide reproducible results in infants and children but requires meticulous attention to detail (Joseph, 1994). Several mechanical factors may adversely influence urodynamics data, creating artifacts that can have a negative impact on the validity of the evaluation if they are not recognized. Testing is typically performed with transurethral catheter placement in this population of patients. The size of the catheter can lead to the appearance of elevated leak or voiding pressure and the inability to empty well, particularly in infants and young boys (Decter and Harpser, 1992). Suprapubic catheter placement circumvents this problem but is not practical in most cases. The testing medium and infusion rate can influence the results. Carbon dioxide is not as reliable as fluid infusion, particularly in evaluation of bladder compliance and capacity. The most common fluids used for testing are saline and iodinated contrast medium, both of which provide reproducible results (Joseph, 1993). Use of testing media at body temperature is also appropriate (Joseph, 1996). End filling pressure, and therefore bladder compliance, can be dramatically affected by simply changing the filling rate (Joseph, 1992). Bauer (1979) has suggested that cystometrography be performed at a fill rate of no more than 10% of the predicted bladder capacity per minute. The bladder neck and external urinary sphincter work in synergy, but only one is required to maintain urinary continence. Often, neurogenic dysfunction leads to abnormalities of both the bladder neck and external urinary sphincter, resulting in diminished outlet resistance during storage or dyssynergic function with voiding. Monitoring of external urinary sphincter electrical activity is required to evaluate coordinated voiding and dyssynergic detrusor-sphincter activity. Perineal surface electrodes, abdominal wall sensors, anal plugs, vaginal monitors, electrical wires, and concentric needle electrodes have all been used for electromyography (Joseph, 1996). In children with neurogenic dysfunction, a concentric needle electrode or dual needle electrodes placed through a 25-gauge needle increase accuracy in measuring sphincter activity (Blaivas et al, 1977; Joseph, 1996). The functional length of the external urinary sphincter also plays a role in outflow resistance, and its measurement can be undertaken with urethral pressure profilometry. Unfortunately, the short length and small diameter of the pediatric urethra make this study technically more difficult to perform than in the adult because the mechanical pulling device is not practical for pediatric use. To assess urethral pressure profile, a constant infusion of testing medium at a rate of 2 mL/min with use of a continuous Harvard pump is required (Joseph, 1996) to eliminate pressure wave artifacts noted with the standard roller ball infusion pumps. Hand withdrawal of the catheter is done while every 5 mm is marked on the recording strip. With practice, reliable measurements can be obtained. There is limited value in comparing specific urethral pressure profile results for a patient against a standard uroflow nomogram; however, a preoperative urethral pressure profile for a given patient provides baseline information that can be beneficial in assessing the intraoperative and postoperative functional urethral length. Some surgeons use leak point pressure to evaluate outflow resistance, the bladder pressure causing leakage per urethra, which can be determined during passive filling and Valsalva maneuver. Fluoroscopy can be informative during those events. The leak point pressure may be artifactually elevated by the urodynamics catheter in a small male urethra (Decter and Harpser, 1992). Much work remains to determine how well such measurable parameters correlate and identify which patients require a procedure on the outlet to achieve adequate continence. The patient’s ability to empty the bladder before reconstruction should be assessed carefully. Useful parameters related to bladder emptying include synergistic relaxation of the external sphincter on electromyography, urinary flow rates, and measurements of postvoid residual urine. Neurologically normal patients who are able to empty the bladder well preoperatively are much more likely to do so after reconstruction than are patients who have neurogenic dysfunction or those who are unable to empty well preoperatively. No test ensures that a patient will be able to void spontaneously and empty well after bladder augmentation or other reconstruction. Therefore, all patients must be prepared to perform CIC postoperatively. The native urethra should be examined for the ease of catheterization. Ideally, the patient should learn CIC and practice it preoperatively until the patient, family, and surgeon are comfortable that catheterization can and will be done reliably. Physical and psychosocial limitations of the patient must be considered in regard to the ability to self-catheterize and to perform self-care. Failure to catheterize and empty reliably after bladder reconstruction may result in upper tract deterioration, urinary tract infection, or bladder perforation despite a technically perfect operation. Most patients who may catheterize per native urethra or an abdominal wall stoma will overwhelmingly prefer the latter (Horowitz et al, 1995). Historically, each patient has undergone preoperative bowel preparation to minimize the potential risk of surgery if the use of any bowel is contemplated. Even when ureterocystoplasty or other alternatives are planned, intraoperative findings may dictate the need for use of a bowel segment. A clear liquid diet for 2 days before bowel preparation aids in clearing solid stool. The patient should then undergo full mechanical bowel preparation the day before surgery. Such bowel preparations have usually been done in the hospital (O’Donnell, 2007); however, recent trends find this changing to an outpatient setting. Major reconstructive procedures often require many hours of operative time with large fluid shifts. It is critical that the patient be well hydrated at the time of surgery. There should be a low threshold for the use of intravenous fluids during the preoperative day. The use of oral antibiotics in bowel preparation, once dogma, is now a matter of personal preference (Breckler et al, 2007). Special attention must be paid to the bowel preparation of patients with neurogenic dysfunction. Most of these patients have chronic constipation. Good bowel cleansing is difficult in such patients and should be done aggressively. Several days of oral cathartics and a clear liquid diet at home may be helpful. Patients with renal insufficiency should be observed carefully during bowel preparation for dehydration and electrolyte disturbances. Theoretically, gastric contents are sterile, and parenteral antibiotics and a routine bowel preparation are not necessary before gastrocystoplasty. In one study (Gundeti et al, 2006) it was suggested that routine bowel preparation may not be necessary before ileocystoplasty in children. All patients should have a urine culture performed several days before bladder reconstruction. The bladder should not be opened with infected urine, which will spill intraperitoneally. Any patient with a positive preoperative urine culture should undergo treatment with either oral or intravesical antibiotics and have a second culture documenting sterile urine before the procedure. Many pediatric patients undergoing augmentation cystoplasty have spina bifida and a ventriculoperitoneal shunt. With a negative urine culture and good bowel preparation the incidence of shunt infection or problems should be low (Yerkes et al, 2001; Hayashi et al, 2008). On occasion, the urinary bladder is so small that it is inadequate for bilateral reimplantation, and alternatives must be considered. It is preferable to reimplant both ureters separately, although if the native urinary bladder is small and adequate for only a single ureteral tunnel, transureteroureterostomy and a single reimplant may be helpful (Fig. 129–1). Typically, the better ureter should be implanted into the bladder, draining the other across into it. The crossing ureter should be mobilized to swing gently across the abdomen to the recipient side in a smooth course without tension. It should be carefully mobilized with all of its adventitia and as much periureteral tissue as possible to preserve blood supply. Care must be taken not to angulate the crossing ureter immediately beneath the inferior mesenteric artery. The crossing ureter is widely anastomosed to the posteromedial aspect of the recipient ureter. The recipient is not mobilized or brought medially to meet the end of the other ureter. Transureteroureterostomy, when it is fashioned appropriately, is successful and carries minimal risk for leakage or obstruction (Hodges et al, 1963; Hendren and Hensle, 1980; Noble et al, 1997; Mure et al, 2000). A history of previous calculi remains a relative contraindication to transureteroureterostomy. Figure 129–1 Technique for transureteroureterostomy. AO, aorta; Inf. mes. art., inferior mesenteric artery. (Courtesy of W. Hardy Hendren.) Fixation of the bladder to the psoas muscle allows precise control of both the length and direction of a ureteral reimplant. Securing the bladder in this manner helps the bladder reach a short ureter or development of a long tunnel when necessary. A psoas hitch should prevent any angulation of the ureter with bladder filling. Such angulation may be particularly problematic or obstructive with a dilated and scarred ureter. The bladder should be secured to the psoas muscle and fascia with nonabsorbable sutures. Those sutures must not enter the bladder lumen or stones will occur. Broad, shallow purchases of the psoas muscle and fascia should be used to hold long term without trapping the sciatic nerve. The hitching sutures should not be tied so tightly that they cut through either bladder or psoas muscle. The contralateral bladder pedicle may be divided to increase bladder mobility and the length of the hitch on occasion. In general, the ureteral reimplantation should be performed before the bladder psoas hitch (Fig. 129–2). (Courtesy of W. Hardy Hendren.) Necessity of ureteral reimplantation into an intestinal segment may occasionally determine the segment to be used for bladder augmentation or replacement. Long experience with ureterosigmoidostomy and colon conduit diversion has established an effective means of antireflux into a colonic segment. A flap valve mechanism can be constructed by tunneling the ureter beneath a taenia. Important principles learned from ureterosigmoidostomy include a direct mucosa-to-mucosa anastomosis and a submucosal tunnel of adequate length. This technique, familiar to most urologists, has provided favorable long-term results since the 1950s (Nesbit, 1949; Goodwin et al, 1953; Leadbetter and Clarke, 1954) and is based on the initial work of Coffey (1911). Implantation may be done from inside the reservoir with the intestinal segment open or from without after the intestinal segment has been completely reconfigured and closed. If a gastric segment is used for bladder augmentation or replacement, ureters may be reimplanted into the stomach in a manner remarkably similar to that used in the native bladder. It is easy to form a submucosal tunnel with good muscle backing. The same principles for choosing the length of tunnel relative to the width of the ureter are used as with bladder. Construction of an effective antireflux mechanism into an ileal segment is more difficult. The split-nipple technique described by Griffith may prevent reflux at least at low reservoir pressure (Turner-Warwick and Ashken, 1967; Patil et al, 1976; Stone and MacDermott, 1989; Sagalowsky, 1995). A short longitudinal incision is made in the distal ureter and the ureteral wall turned back on itself. The nipple should be at least twice as long as the width of the ureter. The cuff is stabilized by suturing the ureter to itself. The adventitia of the ureter immediately proximal to the cuff is then approximated to the full thickness of the ileal wall at the hiatus so that the cuff protrudes into the lumen. Le Duc and associates (1987) described a technique in which the ureter is brought through a hiatus in the ileal wall. From that hiatus the ileal mucosa is incised and the edges are mobilized to construct a trough for the ureter. The spatulated ureter is laid into the trough and approximated to the mucosa at the distal end. The ileal mucosa is sutured to the lateral edges of the ureter and should eventually grow over it. Long-term results with these two techniques have been conflicting but generally have not proved as reliable as a tunneled ureterocolonic anastomosis in preventing reflux (Patil et al, 1976; Le Duc et al, 1987; Rowland, 1996; Bihrle, 1997), with one exception (Lugagne et al, 1997). It is also possible to construct an antirefluxing, serosa-lined tunnel between two limbs of ileum as described by Abol-Enein and Ghoneim (1999; Soygur et al, 2005). Reinforced nipple valves of ileum have been used extensively for antireflux with the Kock pouch (Skinner et al, 1989). After several modifications by Skinner, good long-term results have been achieved. This technique requires a relatively long segment of ileum and use of permanent staples. Attempts have been made to secure the nipple long term without staples or mesh (Hanna and Bloiso, 1987; Gosalbez and Gousse, 1998; Tsuchiya et al, 2004). Maintenance of the intussuscepted cuff is the key to a successful result. The same forces that compress the nipple to achieve antireflux or continence tend to evert or destabilize it. An ileal nipple valve may be particularly useful with short, dilated ureters; an isoperistaltic segment of ileum may be left with the nipple to replace a short ureter. Likewise, the antireflux mechanism is based on the ileal segment and not the ureter. Consequently, a very dilated ureter may be anastomosed to the ileum without tapering. In some neobladders, an isoperistaltic limb of ileum is positioned between the reservoir and ureters to discourage reflux, at least at low pressures (Studer and Zingg, 1997). One of the greatest technical challenges facing the surgeon in bladder reconstruction is providing adequate outflow resistance. The bladder neck may be incompetent due to neurogenic dysfunction secondary to spinal dysraphism. Other underlying pathologic conditions, such as exstrophy, bilateral ectopic ureters, and ureteroceles, are unique problems that must be considered in addressing outlet resistance. Many operative techniques have been described for bladder neck reconstruction, indicating that no single option is best for all patients (Kryger et al, 2000; Cole et al, 2003; Lemelle et al, 2006; Dave et al, 2008a). Khoury and associates (2008) have challenged the need for bladder neck reconstruction in the incontinent neurogenic patient with severe bladder trabeculation. The necessity of preoperative evaluation and thorough knowledge of the patient’s specific physiologic limitations cannot be overstated. The capability for a sustained bladder contraction to result in complete emptying by voiding or the absence of hyperreflexic contractions may influence the technique selected for gaining outlet resistance. Any operative technique that increases outlet resistance may do so at the expense of detrusor contractibility. For reasons not clearly defined, increasing outlet resistance can change the behavior of the detrusor into a noncompliant hostile environment (Bauer et al, 1986; Burbige et al, 1987; Churchill et al, 1987). Provocative cystometry may identify only some patients at risk, and close postoperative observation is mandatory (Kronner et al, 1998b). The following discussion covers a variety of operative techniques used to achieve urinary continence through bladder neck and external urinary sphincter reconstruction. Most of the results are based on experience in individuals with spinal dysraphism, but the techniques may be used with other pathogenic conditions. All techniques have a learning curve, which necessitates careful analysis of results and forthright reporting. An evidence-based review of operative bladder neck procedures found assessment of results to be limited by several factors, including the lack of a true, consistent definition of “success” and “continence,” consideration of patients with and without concomitant bladder augmentation, and evaluation of small populations of patients with mixed pathologic conditions (Joseph et al, 2003). The Young-Dees-Leadbetter bladder neck reconstruction is one of the most recognized operative techniques to increase outlet resistance. The original Young procedure has evolved and remains of primary consideration in reconstruction of the exstrophic bladder neck (Ferrer et al, 2001). It has been used in patients with many diagnoses. Young’s initial description (1919) of excising a portion of the bladder neck and significantly tightening the bladder neck over a silver probe was modified by Dees (1949), who extended the length of excised tissue through the trigone. Leadbetter (1964) followed by elevating the ureters off the trigone, placing them in a more cephalad position on the bladder floor. This allowed tubularization of the trigone and further enhanced lengthening of the urethra. A detailed description and illustrations are found in Chapter 124. Reports of success with the Young-Dees-Leadbetter bladder neck reconstruction in children with neurogenic sphincter dysfunction are limited, not only in the number of patients but also in overall improvement. Tanagho (1981) and Leadbetter (1985) independently reviewed their long-term results and showed minimal success in individuals with neurogenic dysfunction. They speculated that the lack of success was due to a lack of muscle tone and activity in the wrapped muscle related to the underlying neurogenic problem. Many patients in the early series did not undergo bladder augmentation, possibly compromising the continence achieved. Contrary to the results reported in exstrophy patients, the majority of individuals with neurogenic deficiency of the bladder neck will require bladder augmentation and intermittent catheterization. Sidi and colleagues (1987b) documented a 4-hour continence interval in 7 of 11 patients after such repair, although 10 required catheterization and 9 required augmentation. Five of the 7 needed reoperation to achieve continence. This small series represents one of the more recent long-term results of the Young-Dees-Leadbetter reconstruction in children with neurogenic dysfunction because it has largely fallen out of favor. In an attempt to enhance the Young-Dees-Leadbetter procedure, Mitchell and Rink (1983) described the addition of external support and compression achieved through the placement of a silicone sheath around the reconstructed bladder neck. This was somewhat done to establish a plane for future placement of an artificial sphincter cuff, if necessary. In place, it seemed to improve the function of the repair by either improving coaptation or by maintaining the proximal repair in a better anatomic position. Unfortunately, most of the thicker Silastic sheaths eventually eroded and disrupted the repairs (Kropp et al, 1993). Quimby and colleagues (1996) used a thinner Silastic sheath without the same risk of erosion. They also wrapped omentum between the repair and Silastic wrap. The authors reported better results for continence and thought that placement of an artificial sphincter cuff was much easier when needed. Donnahoo and coworkers (1999) reviewed one of the largest series of the repair used in treatment of neurogenic incontinence (38 children, 25 of whom were girls). A primary repair was performed in 24 children, a secondary procedure in 6, and a primary repair in conjunction with a silicone sheath in 8. Partial continence was achieved after the initial repair in 26 children (68%). All children with the silicone sheath were initially continent, but erosion occurred in 5. More critically, 35 (92%) of the children required augmentation cystoplasty to become continent. The authors found that although continence could be achieved with this technique, it was at the expense of augmentation cystoplasty and multiple procedures. Their results are similar to those reported by Cole and associates (2003). Sling procedures were developed in an attempt to increase resistance at the bladder neck. Both artificial and natural tissue have been used with similar technique. Resultant coaptation and elevation of the bladder neck should cause approximation of opposing epithelial surfaces and increased outlet resistance that is greater than the resting bladder pressure and the pressure achieved during stressful activity or Valsalva behavior. With sling coaptation, the bladder neck remains fixed; and although a strong detrusor contraction can establish a voiding pressure leading to urine flow, it rarely allows adequate bladder emptying in the face of anatomic or neurologic problems. The majority of pediatric patients who undergo a sling procedure must be prepared for intermittent catheterization. The resistance achieved with bladder neck slings can potentially be overcome by hyperreflexic bladder contractions or elevated pressure due to diminished bladder compliance. Therefore simultaneous bladder augmentation has again been reported in 55% to 100% of patients who achieve urinary continence after a sling procedure (Bauer et al, 1989; Elder, 1990; Decter, 1993; Kakizaki et al, 1995; Perez et al, 1996a; Dik et al, 1999, 2003; Walker et al, 2000; Bugg and Joseph, 2003; Cole et al, 2003; Godbole et al, 2003). Snodgrass and associates (2007) have reviewed their series of 30 consecutive patients with neurogenic incontinence who underwent bladder neck fascial sling only. They conclude continence can be achieved without upper tract damage and the adverse effect of enterocystoplasty. Others have cautioned taking this approach (Dave et al, 2008b). Alternatives to fascia, such as an expanded fluorocarbon polymer (Gore-Tex), have been used in a similar fashion, although early continence has not been maintained (Godbole and Mackinnon, 2004). Good early results have been noted with small intestinal submucosa and other biodegradable scaffolds (Colvert et al, 2002; Misseri et al, 2005). The bladder neck is exposed by clearing fatty tissue overlying the bladder neck and the lateral endopelvic fascia. An incision is made within the endopelvic fascia for approximately 2 cm. The junction between the bladder neck and proximal urethra can be identified by placing a transurethral catheter into the bladder and gently pulling down on the catheter to lodge the balloon at the bladder neck. By blunt dissection, a plane between the posterior bladder neck and vagina in girls or rectal wall in boys is developed. The proper plane may be more easily developed from the cul-de-sac by dissecting behind the bladder and ureters from above (Lottmann et al, 1999; Badiola et al, 2000). If the landmarks are not easily defined, as in a secondary repair, the dissection becomes difficult. It may be appropriate to open the bladder to help prevent inadvertent dissection into the urethra or posterior structures. Storm and associates (2008) reported on a robotic-assisted laparoscopic approach for posterior bladder neck dissection and placement of the sling. This may become more appealing as robotic technology expands into bladder reconstruction. Dik and colleagues (2003) proposed a transvaginal approach, eliminating the need to open the bladder or to dissect between the bladder neck and anterior vagina. When fascial tissue is used the technique is based on that described by McGuire and Lytton (1978) for stress urinary incontinence. Rectus abdominis fascia 1 cm in width and an appropriate length is harvested. This fascia can be taken in either vertical or horizontal fashion, depending on the initial skin incision. Fascia from other sites has been used in a similar fashion but requires a second incision. Often poor nutritional states and prior abdominal procedures limit the availability of rectus fascia, resulting in the use of alternative material such as small intestinal submucosa, cadaveric tissue, biodegradable scaffolds, and bladder wall (Colvert et al, 2002; Misseri et al, 2005; Albouy et al, 2007). All are generally secured to the anterior rectus fascia on either side. Autologous fascial tissue has been used, combining the benefits of a compressive wrap and suspension of the proximal urethra and bladder neck. Several variations of fascial placement and configuration have been described (Woodside and Borden, 1982; McGuire et al, 1986; Elder, 1990; Perez et al, 1996a; Bugg and Joseph, 2003; Dik et al, 2003). When fascial slings and wraps are used for neurogenic sphincter incontinence there is not as much concern for making a wrap or sling that is too tight because most patients are preferentially managed with CIC. Fascial slings have been used more extensively and with better results in girls with neurogenic sphincter incompetence, although some success has recently been reported in boys. Overall long-term success with fascial slings in the neurogenic population has varied greatly from 40% to 100% (Kryger et al, 2000; Castellan et al, 2005; Misseri et al, 2005; Snodgrass et al, 2007). A variation thought to contribute to a higher success includes a circumferential fascial wrap around the bladder neck. A circumferential wrap may equalize the compressive pressure over a greater surface area of bladder neck and posterior urethra (Walker et al, 1995; Strang et al, 2006). With a 360-degree wrap, simultaneous suspension has also been applied (Bugg and Joseph, 2003). Success rates have varied so much that it is difficult to determine whether any modification of the sling accounts for an increase in continence. Most patients who have undergone a fascial sling or wrap have also had simultaneous bladder augmentation. Success of the sling, as with most repairs, appears to be improved with augmentation cystoplasty in this patient population by almost all reports (Castellan et al, 2005). Perez and associates (1996a) reviewed the outcome of sling cystourethropexy in 39 children, 15 of whom were boys. One of four techniques was performed. When postoperative continence was evaluated on the basis of age, sex, underlying diagnosis, preoperative urodynamics, surgical technique, and enterocystoplasty, only concomitant enterocystoplasty was predictive of a successful outcome. Contrary to the Silastic sheath, fascial sling erosion rarely occurs. Gormley and coworkers (1994) reported a revision rate with fascial slings of 15%. Placement of a fascial sling does not eliminate the possibility of later placement of an artificial urinary sphincter (Decter, 1993; Barthold et al, 1999). It is not unreasonable to consider placement of a fascial sling in a child with a marginally competent bladder neck and posterior urethra if the child is undergoing augmentation cystoplasty and already requires intermittent catheterization. Vorstman and colleagues (1985) reported one of the initial descriptions of injection therapy with a bulking agent into the bladder neck of incontinent children. The initial enthusiasm for use of polytetrafluoroethylene was quickly tempered because of concern about migration of particles to regional and distant sites including pelvic nodes, lungs, brain, kidney, and spleen found in animal models (Malizia et al, 1984). The technique remains of interest, and several alternatives to polytetrafluoroethylene have been assessed, including glutaraldehyde cross-linked collagen, dextranomer/hyaluronic acid copolymer, and polydimethylsiloxane (Leonard et al, 1990a; Guys et al, 2006; Knudson et al, 2006; Lottmann et al, 2006; Dean et al, 2007). Bovine collagen should not be used in latex-sensitive children with spina bifida because the product is not latex free (Kryger et al, 2000). In an attempt to achieve an ideal substance for injection, investigation is ongoing with autologous cartilage cells harvested from a separate site and then grown in an alginate matrix for endoscopic implantation (Bent et al, 2000). Although preliminary results have been obtained in adult women with stress incontinence, the material has not achieved an enthusiastic use in pediatrics. Whether this or other materials will be an appropriate alternative for neurogenic sphincter incontinence in children is yet to be seen. In an attempt to alleviate the risks with injection of a foreign biologic product, alternatives to collagen and bovine have been investigated. Polydimethylsiloxane is one such agent. It is composed of sterile solid textured silicone particles, with an average size of 200 µg, suspended in a biologic hydrogen carrier. The large size of the particles should virtually eliminate lymphatic and distant migration (Beisang and Ersek, 1992; Guys et al, 1999; Halachmi et al, 2004; Guys et al, 2006). Contemporary reports favor the use of dextranomer/hyaluronic acid (Lottmann et al, 2006; Dean et al, 2007; Dyer et al, 2007). Endoscopic exposure is used to localize the proximal urethra and bladder neck. Injection can be done directly through the working channel of the endoscope, often one with an offset lens system. Ideal placement of the material is in a subepithelial space, mobilizing the epithelium toward the lumen of the bladder neck. When it is completed in a circumferential fashion, adequate epithelial coaptation may occur that effectively raises outlet resistance. Alternatively, periurethral injection in women with a long needle placed from the perineum or through a suprapubic approach has been used. Dean and associates (2007) advocate simultaneous antegrade and retrograde endoscopic injection. Evidence is lacking whether the exact approach affects success, but accurate placement is important and transurethral injection generally preferred. The durability and success of bladder neck and proximal urethral injection remain in doubt for the pediatric population, particularly those with neurogenic dysfunction. True continence, as defined by a 4-hour dry period between voidings or catheterization, has been reported to be at most 64% and has ranged as low as 5% (Leonard et al, 1990a; Capozza et al, 1995; Bomalaski et al, 1996; Perez et al, 1996b; Sundaram et al, 1997; Silveri et al, 1998; Guys et al, 2001, 2006; Godbole et al, 2003; Halachmi et al, 2004; Lottmann et al, 2006; Dean et al, 2007). Several factors play a role in the outcome, one of which is a history of any previous operative bladder neck repair. Success is enhanced by elevation of the epithelium of the bladder neck, which may be compromised by scarring from previous operative procedures. The concept of a minimally invasive operation used to enhance a marginal result gained from a more formal bladder neck repair is enticing; unfortunately, the data are lacking to show that bladder neck injection is of lasting value in that setting. Sundaram and colleagues (1997) reported on the efficacy and durability of glutaraldehyde cross-linked bovine collagen in 20 children, 12 of whom had neurogenic sphincter dysfunction. More than half of the children required two or three independent injections. Success was achieved in only 1 patient (5%), who was considered dry; 5 had some improvement, and 10 had either no change or transient improvement of only 2 to 90 days. Thus collagen therapy only delayed the ultimate need for bladder neck reconstruction. Submucosal bladder neck injection of bovine dermal collagen was used by Perez and coworkers (1996b) in 32 patients. Continence was achieved after a single injection in only 20% of the children with neurogenic dysfunction. Complications were limited to febrile urinary infections, one episode of urinary retention, and worsening incontinence in 2 patients. The authors concluded that even though their success was limited the low morbidity and ease of placement justified submucosal injection in selected children. Guys and associates (2006) treated 49 children with polydimethylsiloxane, 41 of whom had neurogenic bladder neck and sphincter incontinence. The level of continence continued to deteriorate through 18 months, then stabilized. At an average follow-up of 73 months, success was achieved in 16 (33%) and 7 (14%) were improved. Godbole and colleagues (2003), Halachmi and associates (2004), and Dyer and coworkers (2007) independently came to the conclusion that regardless of the material injected (polytetrafluoroethylene, collagen, polydimethylsiloxane, dextranomer/hyaluronic acid), short-term success is not long lasting. Kitchens and colleagues (2007) evaluated the use of dextranomer/hyaluronic acid to correct incontinence in patients who had previously undergone bladder neck reconstruction. They achieved success or improvement in 70% of select patients at 17 months, indicating this may be an alternative to other salvage therapy. Overall, the cost of the bulking agents can be excessive and there does not appear to be any financial benefit over a formal repair (Kryger et al, 2000). At present, bulking agents play a limited role for increasing outlet resistance and should be reserved for a select group of patients. The exact criteria that define that group have not been established. Patients with marginal native outflow resistance are probably better candidates than are those with minimal preoperative function. The artificial urinary sphincter has been recognized as a device that can result in prompt continence in select children while preserving their ability to void spontaneously. The artificial urinary sphincter was introduced by Scott in 1974. The general concept and design of the initial model have been retained; however, improvements and enhancements have evolved that have positive impact on the long-term success of the artificial urinary sphincter. The current 800 model includes a seamless, pressurized balloon reservoir, nonkink tubing, and changes in the cuff that facilitate its placement and effectiveness with coaptation of the bladder neck and proximal urethra (Light and Reynolds, 1992; Barrett et al, 1993; Lai et al, 2007). Alternatives to the AS800 model have also been explored (Vilar et al, 2004). Placement of the cuff should be at the level of the bladder neck in all female patients and prepubertal boys (Fig. 129–3). It is also the most desirable and effective location in pubertal boys and adult men with neurogenic sphincter incompetence. The bulbar urethra can be used as an alternative site in adult men with mature spongiosum. Levesque and associates (1996) have indicated that age is not a factor in placement of the cuff around the bladder neck. They found that children do not outgrow the artificial urinary sphincter as they progress through puberty and that replacement of the cuff is not routinely necessary. The artificial urinary sphincter cuff can be positioned around intestinal segments used in total urinary reconstruction but is more susceptible to erosion there. Several authors have described the successful placement of the cuff around a bowel segment, particularly when omentum is interposed between the cuff and the segment (Burbige et al, 1987; Weston et al, 1991; Light et al, 1995). Placement of an artificial sphincter in children is the same as that described for adults. Development of the proper plane for the cuff is virtually identical to that described for a fascial sling. The cuff should be sized snugly, but not tightly, around the bladder neck. Obviously, a sterile environment is critical in considering placement of the artificial urinary sphincter to avoid infection. For that reason, preoperative antibiotics are a necessity, and confirmation of sterile urine is required. With those precautions there is freedom to open the bladder in dissecting around the bladder neck. This will often facilitate the dissection and ensure proper placement. The AS800 model has a locking mechanism in the pump that permits the artificial urinary sphincter to be deactivated and activated without a second operative procedure. Experience has shown that leaving the unit deactivated with the cuff deflated after placement allows formation of a pseudocapsule around the cuff and decreases the risk of erosion (Furlow, 1981; Sidi et al, 1984). The noncycled artificial urinary sphincter occasionally may provide enough resistance for continence, eliminating the disadvantages of activation (Herndon et al, 2004a). There are substantial short- and long-term data regarding continence after placement of the artificial urinary sphincter, with the largest series presented by Herndon and associates (2003). In investigation of continence, it must be placed in context of the cost experienced by the patient defined by mechanical malfunctions resulting in secondary operative procedures and more catastrophic complications, such as device infection and erosion. Dramatic improvement regarding the need for secondary procedures has occurred with the technical refinements in the device. Ten- to 15-year long-term follow-up of the artificial urinary sphincter in children has been reported (Levesque et al, 1996; Kryger et al, 1999, 2001; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003; Lopez Pereira et al, 2006; Ruiz et al, 2006). All groups report an impressive continence rate of 80% and a functioning sphincter in 95% of patients. These reports are consistent with older series in children reporting continence rates of 75% to 90% and a functioning sphincter in 85% to 97% (Nurse and Mundy, 1988; Gonzalez et al, 1989a; Bosco et al, 1991; O’Flynn and Thomas, 1991; Aprikian et al, 1992; Singh and Thomas, 1996; Simeoni et al, 1996; Catti et al, 2008). Herndon and colleagues (2003) presented the most comprehensive long-term data. They achieved overall continence in 86% of 142 patients with an average follow-up of 10 years. Age at implementation does not appear to affect continence (Kryger et al, 2001). Whereas the artificial urinary sphincter is one of the few surgically created continence mechanisms that does not negatively affect spontaneous voiding, intermittent catheterization remains an important adjunct in approximately 75% of children with neurogenic sphincter incompetence and can be performed successfully through the cuff (Diokno and Sonda, 1981; Gonzalez et al, 1995; Levesque et al, 1996; Kryger, 1999, 2001; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003). As boys approach puberty, spontaneous voiding may become progressively inadequate. It has been speculated that growth of the prostate causes an increase in native outlet resistance. Kaefer and associates (1997a) evaluated increases in cuff size to facilitate spontaneous voiding in boys. In their limited series, they did not find that up-sizing restored the ability to void spontaneously. Jumper and colleagues (1990) reported on prostatic development and sexual function in pubertal boys with spinal dysraphism who had been treated with the artificial urinary sphincter. They found that the artificial sphincter did not alter sexual development, prostatic growth, or morphologic features. Herndon and associates (2003) reported device malfunction in 64% with the pre-AS800 model and 30% with the AS800. Sphincter erosion was similar for the pre-AS800 and AS800, occurring at 19% and 16%, respectively, in their experience. Fastidious attention to detail and sterile technique diminish the risk of infection but do not eliminate it. When infection occurs without erosion, the unit can be removed and later replaced (Nurse and Mundy, 1988). Infections are minimized by sterilization of the urine preoperatively, meticulous cleaning of the wound site, preoperative bowel preparation, perioperative parenteral antibiotics, and copious antibiotic wound irrigation. Newer cuff design and a 6-week delay in activation of the device help formation of a thickened pseudocapsule that substantially decreases bladder neck and proximal urethral erosion. Kryger and associates (1999) indicated that erosion can be virtually eliminated when the cuff is placed as the primary treatment for bladder neck incompetence. They and others (Aliabadi and Gonzalez, 1990; Gonzalez et al, 1995; Simeoni et al, 1996; Levesque et al, 1996; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003) noted that the risk of erosion substantially increases after previous failed repairs but this has not been the experience of others (Ruiz et al, 2006). Identification of the correct plane between the bladder neck and vagina in female patients or rectum in male patients preserves the vascularity of the bladder neck and proximal urethra and may decrease the rate of erosion (Aliabadi and Gonzalez, 1990). Initial exposure through a posterior bladder approach as described by Lottmann and coworkers (1999) may be helpful. Shankar and colleagues (2001) suggested that there is an advantage to exposure of the bladder neck with a transperitoneal approach by decreasing the potential of bleeding from the prostatic venous plexus and improving visualization of the rectal wall. Levesque and associates (1996) evaluated the long-term outcome of the artificial urinary sphincter on the basis of date of insertion and location of the placement. Before 1985 the artificial urinary sphincter had been inserted in 36 children. Between 1985 and 1990, an additional 18 children underwent placement. In the original group, 24 of the 36 sphincters were in place and 22 functional; 12 had required at least one revision. The mean survival of the device was 12.5 years. Success rates at 5 and 10 years were 75% and 72%, respectively. In the group implanted after 1985, 78% retained a functioning sphincter. The overall continence rate in both groups was 59%; sphincter survival probability at 10 years was approximately 70%. There was no difference found between failure rates in males and females with the exception that female patients who had previously undergone bladder neck surgery were more likely to suffer an erosion. The ability to void independently without the use of intermittent catheterization was retained in 36 children (67%). Those findings are supported by contemporary series (Levesque et al, 1996; Kryger, 1999, 2001; Castera et al, 2001; Hafez et al, 2002; Herndon et al, 2003; Lopez Pereira et al, 2006; Ruiz et al, 2006; Catti et al, 2008). Upper urinary tract changes including hydronephrosis have been reported to occur in up to 15% of children after placement of the artificial urinary sphincter (Light and Pietro, 1986; Churchill et al, 1987; Gonzalez et al, 1995; Levesque et al, 1996; Kryger et al, 1999). In extreme cases, renal insufficiency has resulted. It is now recognized that occlusion of the bladder neck in children with neurogenic sphincter incompetence can result in the unmasking or development of detrusor hostility manifested by a decrease in bladder compliance or increase in detrusor hyperreflexia (Bauer et al, 1986). Careful preoperative urodynamic assessment helps identify only some of the children who are at risk (Kronner et al, 1998b). When hostile bladder characteristics are found preoperatively, anticholinergic medications can be beneficial for hyperreflexic contractions but augmentation cystoplasty is usually required for diminished compliance. Churchill and associates (1987) showed that favorable parameters can be maintained after placement of the artificial urinary sphincter; however, close observation is still recommended in any child undergoing bladder neck reconstruction to identify any early deterioration in bladder dynamics before upper tract changes. Some children undergoing sphincter placement need bladder augmentation as well, and the timing of the two procedures may be questioned because of the concern for artificial urinary sphincter infection. Light and colleagues (1995) reported a 50% infection rate with simultaneous augmentation compared with 9.5% when the procedures were staged. On the contrary, a contemporary review by Miller and coworkers (1998) found that infection necessitating removal of the device occurred in only 2 of 29 such patients (7%). This low rate is similar to that noted by others (Strawbridge et al, 1989; Gonzalez et al, 1989b). Several reports have evaluated various factors and found that the intestinal segment selected for augmentation appeared to be the only parameter affecting results; gastric augmentation was the least offensive regarding infection (Ganesan et al, 1993; Miller et al, 1998; Holmes et al, 2001). Gonzalez and associates (2002) reported an alternative technique using a seromuscular colocystoplasty and simultaneous placement of the artificial urinary sphincter. They achieved continence in 89% without the need for additional procedures and no deterioration of the upper urinary tract. The AS800 is the subject of most reviews when the artificial urinary sphincter is discussed, but alternative devices have been reported (Lima et al, 1996; de O Vilar et al, 2004). An alternative device is a one-piece adjustable cuff connected to an injection port for inflation. The injection port is placed subcutaneously and made available for percutaneous access. This allows for adjusting the pressure within the cuff in order to achieve continence. It is particularly convenient for individuals with limited ability to actively pump the AS800. The periurethral constrictor is wider and more fluid than the AS800. It can be deactivated and reactivated by tapping the subcutaneous port. Young’s original description of bladder neck reconstruction (1919) consisted of two components: excision of a segment of anterior urethral bladder neck tissue and narrowing of the adjacent remaining posterior portion. This, however, ultimately led to failure because the tubularized segment remained unsupported within the bladder. Refinements by Dees (1949) and Leadbetter (1964) maximized good muscle tone at the bladder neck and extension of the urethral tube through the trigone. With similar principles, Tanagho (1981)
The “Functional” Urinary Tract
Basic Bladder Function
Passive: Storage
Active: Voiding
Dysfunction
Upper Tracts
Bladder Dysfunction
Other Considerations
Evaluation of the Patient
Urodynamics
Bladder Dynamics: Capacity and Compliance
Sphincter Dynamics: Outflow Resistance
Bladder Emptying
Preparation of the Patient
Bowel Preparation
Urine Culture
Antireflux
Transureteroureterostomy and Single Ureteral Reimplantation
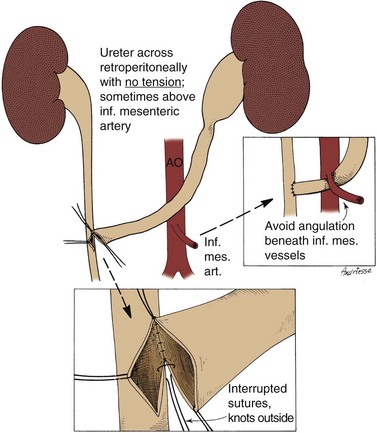
Psoas Hitch
Antireflux with Intestinal Segments
Bladder Neck Reconstruction
Young-Dees-Leadbetter Repair
Technique
Results
Fascial Sling
Technique
Results
Bladder Neck Bulking Agents
Technique
Results
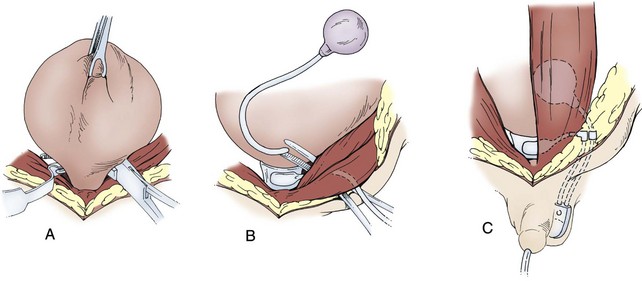
Artificial Urinary Sphincter
Technique
Results
Urethral Lengthening
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Urinary Tract Reconstruction in Children