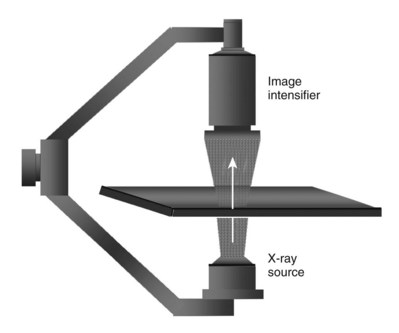
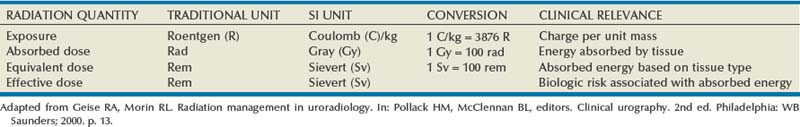
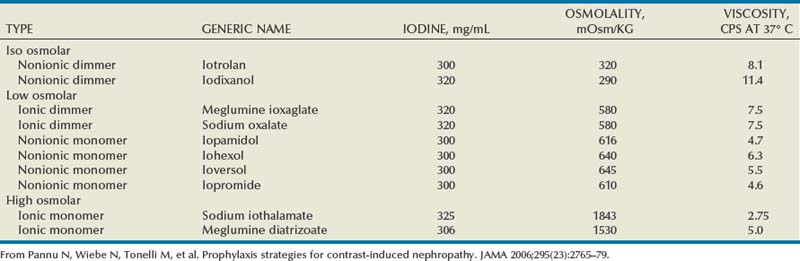
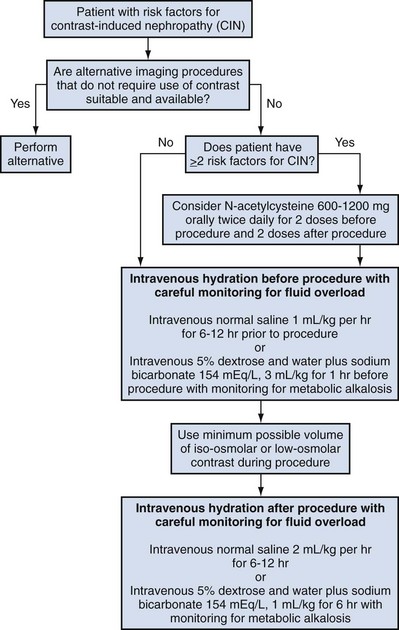
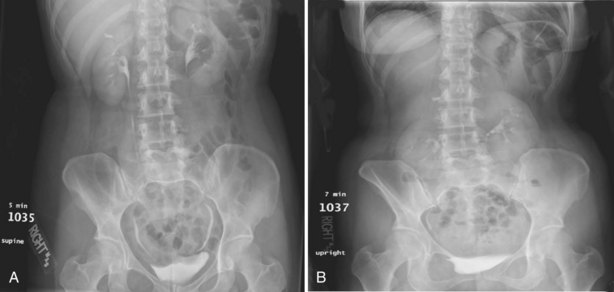
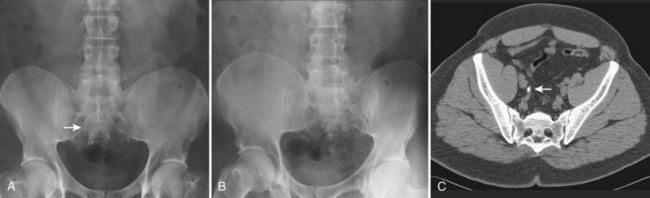
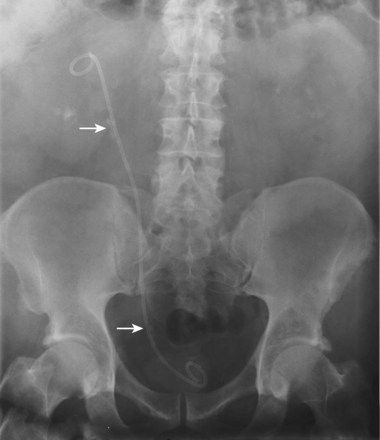
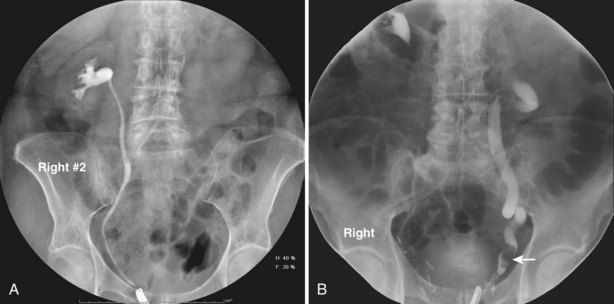
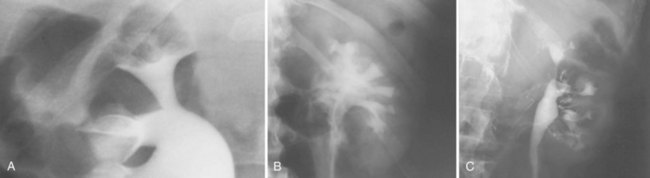
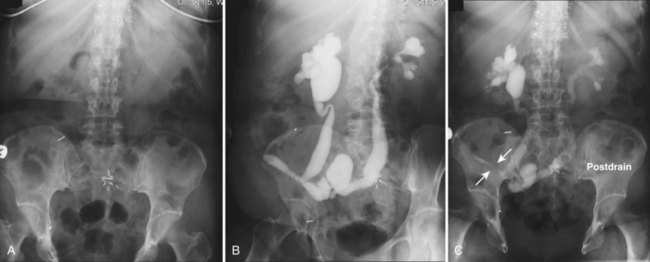
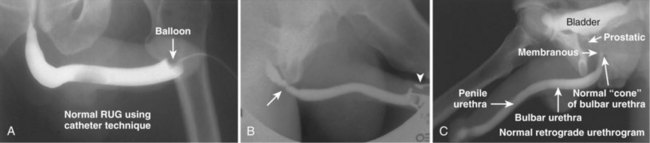
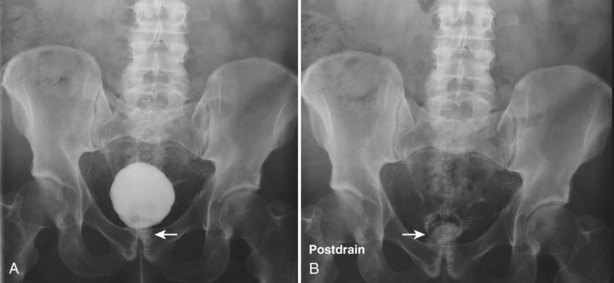
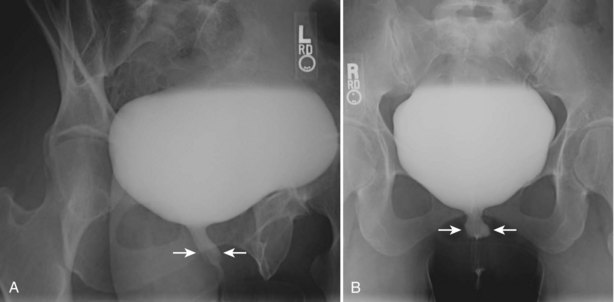
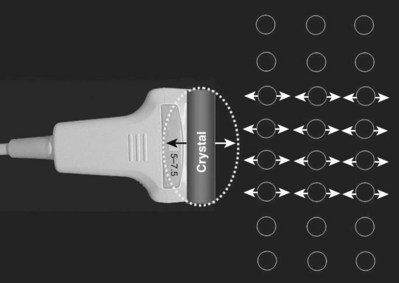
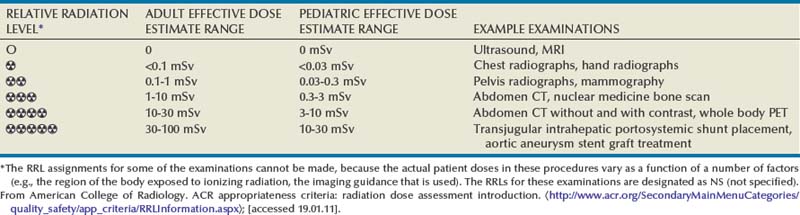
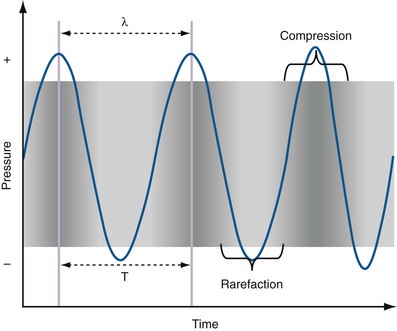
Pat F. Fulgham, MD, DABU, FACS, Jay Todd Bishoff, MD, FACS It is imperative that urologists understand the physics of conventional radiography and fluoroscopy, as well as the implications of radiation exposure to the patient and the operator. The underlying physical principles of conventional radiography involve emitting a stream of photons from an x-ray source. These photons travel through the air and strike tissue, imparting energy to that tissue. Some of the photons emerge from the patient with varying amounts of energy attenuation and strike an image recorder such as a film cassette or the input phosphor of an image-intensifier tube, thus producing an image (Fig. 4–1). The distribution of energy absorption in the human body will be different on the basis of the body part being imaged and various other factors. The most important risk of radiation exposure from diagnostic imaging is the development of cancer. The effective dose is a quantity used to denote the radiation risk (expressed in sieverts) to a population of patients from an imaging study. See Table 4–1 for a description of the relationship between these measures of radiation exposure. The average person living in the United States is exposed to 1 to 3 mSv of radiation per year from ambient sources such as radon and cosmic rays. The recommended occupational exposure limit to medical personnel is 50 mSv per year (NCRP, 1993). Exposure to the eyes and gonads has a more significant biologic impact than exposure to the extremities, so recommended exposure limits vary according to the body part. However, there is no safe dose of radiation. An effective radiation dose of as little as 10 mSv may result in the development of a malignancy in 1 of 1000 individuals exposed (NRCNA, 2006). The assessment of biologic risk from radiation exposure is complex. By estimating the range of effective doses for various imaging modalities, they can be assigned a relative radiation level (RRL) (Table 4–2). Table 4–2 Relative Radiation Level Designations along with Common Example Examinations for Each Classification The effective dose from a three-phase computed tomography (CT) scan of the abdomen and pelvis without and with contrast may be as high as 25 to 40 mSv. An often overlooked source of significant radiation exposure is fluoroscopy. Fluoroscopy for 1 minute results in a radiation dose to the skin equivalent to 10 times that of a single radiograph of the same anatomic area (Geise and Morin, 2000). Key Points Conventional Radiography/Radiation Management in Uroradiology Iodine is the most common element in general use as an intravascular radiologic contrast medium (IRCM). With an atomic weight of 127, iodine has radiopacity, whereas other elements included in IRCM have no radiopacity and act only as carriers of the iodine elements, increasing solubility and reducing toxicity. Four basic types of iodinated IRCM are available for clinical use: ionic monomer, nonionic monomer, ionic dimer, and nonionic dimer. They can be further characterized as being iso-, hyper-, or low-osmolar compared with physiologic osmolality of 300 mOsm/kg H2O (Table 4–3). Relative to the body’s iron stores, large quantities of iodine are required for imaging enhancement. The total body iodine content, found mainly in the thyroid gland, is 0.01 g and the average daily turnover of iodine is only 0.0001 g. For renal CT imaging a common dose of IRCM will expose the patient to between 25 and 50 g of iodine, which is approximately 400,000 times the daily turnover rate in the human body, but this dose will rarely cause any toxicity or lasting effects (Morris, 1993). The exact mechanism of IA reactions is not known but is thought to be a combination of systemic effects. IA reactions have not been shown to result from a true IgE antibody immunologic reaction to the contrast media (Dawson, 1999). At least four mechanisms may play a role in IA reactions: (1) release of vasoactive substances including histamine; (2) activation of physiologic cascades including complement, kinin, coagulation, and fibrinolytic systems; (3) inhibition of enzymes including cholinesterase, which may cause prolonged vagal stimulation; and (4) the patient’s own anxiety and fear of the actual procedure. IA reactions are not dose dependent. Severe reactions have been reported after only 1 mL injected at the beginning of the procedure and have also occurred after completion of a full dose despite no reaction to the initial test dose (Nelson et al, 1988; Thomsen et al, 1999; ACR, 2008). The hyperosmolar contrast media (HOCM) have an osmolality that is five times greater than physiologic osmolality of body cells (300 mOsm/kg water). The hyperosmolar agents are associated with erythrocyte damage, endothelial damage, vasodilation, hypervolemia, interruption of the blood-brain barrier, and cardiac depression. Chemotoxic reactions to IRCM include cardiac, vascular, neurologic, and renal toxicity. The low osmotic contrast media (LOCM) have an osmolality the same as or slightly higher than physiologic osmolality and are associated with fewer ADRs and toxic events (Morcos, 1999). All contrast ADRs are more common with HOCM (12%) compared with LOCM (3%) (Katayama et al, 1990; Heinrich et al, 2005). Previous adverse reaction to IRCM and a history of asthma are two of the most concerning predisposing conditions predicating an ADR with use of contrast media. Additional factors that may predict an ADR include history of known allergy to iodine, severe cardiac disease, renal insufficiency, dehydration, sickle cell anemia, anxiety, apprehension, hyperthyroidism, and presence of adrenal pheochromocytoma. Life-threatening reactions occur in approximately 1/1000 uses for high-osmolar agents and are far less for low-osmolar contrast media, with both types of agents resulting in mortality rates of 1/170,000 uses (Spring et al, 1997). Severe reactions include seizure, laryngeal spasm, bronchospasm, pulmonary edema, cardiac arrhythmia, respiratory collapse, or cardiac arrest (Katayama et al, 1990). Ready access to a crash cart and trained personnel are necessary components of any imaging center. If needed, cardiopulmonary resuscitation is started immediately. Many different medications have been used for the treatment of an acute severe life-threatening reaction to IRCM. However, rapid administration of epinephrine is the treatment of choice for severe contrast reactions. Current guidelines recommend immediate delivery of 0.01 mg/kg of body weight to a maximum of 0.5 mg of 1 : 1000 concentration of epinephrine, injected IM in the lateral thigh as first-line treatment. Subcutaneous injection is much less effective. Intravenous injection can be given with more dilute concentration and must be given slowly (ACR, 2008; Lightfoot et al, 2009). No known premedication strategy will eliminate the risk of a severe adverse reaction to IRCM. The regimens suggested in the literature include the use of corticosteroids, antihistamines, H1 and H2 antagonists, and ephedrine. Patients at high risk should be premedicated with corticosteroids and possibly antihistamines 12 to 24 hours before and after use of IRCM. LOCM can be used in these patients. Several premedication regimens have been proposed to reduce the frequency and/or severity of reactions to contrast media. Two frequently used regimens are outlined in Table 4–4. Table 4–4 Premedication Strategies to Reduce Severity of Reactions to Contrast Media From American College of Radiology Manual on Contrast Media, version 6, pp. 9-10, <http://www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual.aspx> [accessed 01.10.10]. It has been demonstrated that the use of nonionic contrast media combined with a premedication strategy including corticosteroids results in a reduction in reaction rates compared with other protocols for patients who have experienced a prior contrast media–induced reaction. However, no controlled studies are available to determine whether pretreatment alters the incidence of serious reactions. Oral administration of steroids seems preferable to intravascular administration, and prednisone and methylprednisolone are equally effective. If the patient is unable to take oral medication, 200 mg of hydrocortisone IV may be substituted for oral prednisone. One consistent finding is that the steroids should be given at least 6 hours before the injection of contrast media regardless of the route of steroid administration. It is clear that administration for 3 hours or less before contrast does not decrease adverse reactions (Lasser, 1988). Patients with type II diabetes mellitus on metformin oral biguanide hyperglycemic therapy may have an accumulation of the drug after administering IRCM, resulting in biguanide lactic acidosis presenting with vomiting, diarrhea, and somnolence. This condition is fatal in approximately 50% of cases (Wiholm, 1993). Biguanide lactic acidosis is rare in patients with normal renal function. Consequently, in patients with normal renal function and no known comorbidities there is no need to discontinue metformin before IRCM use, nor is there a need to check creatinine following the imaging study. However, in patients with renal insufficiency, metformin should be discontinued the day of the study and withheld for 48 hours. Postprocedure creatinine should be measured at 48 hours and metformin started once kidney function is normal (Bailey and Turner, 1996). It is not necessary to discontinue metformin before gadolinium-enhanced magnetic resonance studies when the amount of gadolinium administered is in the usual dose range of 0.1 to 0.3 mmol per kg of body weight. Contrast-induced nephropathy (CIN) is defined as a rise in serum creatinine 25% above baseline, or more than 0.5 mg/dL within 3 days following exposure to contrast media, in the absence of an alternative cause. The precise cause of CIN continues to elude investigators but is believed to be a combination of tubular injury and renal ischemia (Katholi et al, 1998). High doses of IRCM can impair renal function in some patients for 3 to 5 days. CIN in patients with normal kidney function is rare (Pannu et al, 2006; Kelly et al, 2008). CIN is the third most common cause of acute kidney failure in the hospitalized patients (Nash et al, 2002). The most common patient-related risk factors are chronic kidney disease (creatinine clearance <60 mL/min), diabetes mellitus, dehydration, congestive heart failure, age, hypertension, low hematocrit, and ventricular ejection fraction less than 40%. The patients at highest risk for developing CIN are those with both diabetes and preexisting renal insufficiency. The most common non–patient-related causes are high-osmolar contrast agents, ionic contrast, increased contrast viscosity, and large-contrast volume infusion (Pannu et al, 2006). Despite significant discussion on the part of radiologists and urologists, the literature does not support an absolute serum creatinine level that prohibits the use of contrast media. Prevention of CIN has been the subject of many research studies, and the results have been summarized by several different meta-analyses. In these meta-analyses the baseline serum creatinine of study participants ranged from 0.9 to 2.5 mg/dL. In one survey the policies regarding the cutoff value for serum creatinine varied widely among radiology practices. Thirty-five percent of respondents used 1.5 mg/dL, 27% used 1.7 mg/dL, and 31% used 2.0 mg/dL (mean, 1.78 mg/dL) as a cutoff value in patients with no risk factors other than elevated creatinine; threshold values were slightly lower in diabetics (mean 1.68 mg/dL). Patients in end-stage renal disease who have no remaining natural renal function are no longer at risk for CIN and may receive LOCM or IOCM (Elicker et al, 2006). The summary of the meta-analysis for the prevention of CIN after contrast media use supports using hydration, bicarbonate, iso- or low-osmolar contrast media, and N-acetylcysteine. In one review article, N-acetylcysteine was determined to be more protective than hydration alone. Furosemide was found to increase the risk of developing CIN (Pannu et al, 2006; Kelly et al, 2008). N-acetylcysteine is inexpensive, readily available, administered orally, and associated with few drug interactions or side effects. Its mechanism of protection against CIN is not understood but may serve as a scavenger of oxygen-free radicals and/or augment the vasodilatory effects of nitric oxide in the kidney (Safirstein et al, 2000). Doses used in the different studies ranged from 600 to 1200 mg orally twice a day for 2 doses before the contrast-enhanced study and 2 doses after the procedure (Fig. 4–2). Although gadolinium chelates can be differentiated on the basis of stability, viscosity, and osmolality, they cannot be differentiated on the basis of efficacy. Acute adverse reactions are encountered less frequently with gadolinium than after administration of iodinated contrast media. The frequency of all acute adverse events after an injection of 0.1 or 0.2 mmol/kg of gadolinium chelate ranges from 0.07% to 2.4%. The vast majority of these reactions are mild including coldness at the injection site, nausea, emesis, headache, warmth or pain at the injection site, paresthesias, dizziness, and itching. Reactions resembling an “allergic” response occur frequently, from 0.004% to 0.7%. Systemic reactions consisting of rash, hives, or urticaria are the most frequent of this group, and rarely bronchospasm. The severe, life-threatening anaphylactoid or nonallergic anaphylactic reactions are exceedingly rare (0.0001% to 0.001%). In a meta-analysis of 687,000 gadolinium doses for MRI, there were only five severe reactions. In another survey based on 20 million administered doses there were 55 cases (0.0003%) of severe reactions. Fatal reactions to gadolinium chelate agents have been reported, but they are extremely rare events (Murphy et al, 1999). In 1997 NSF was described in dialysis patients who had not been exposed to GBCM. The condition was previously known as nephrogenic fibrosing dermopathy. In 2006 and again in 2007 independent reports surfaced defining a strong association with gadolinium-based contrast media (Grobner, 2006; Marckmann et al, 2006). Onset of NSF varies between 2 days and 3 months. Early manifestations include subacute swelling of distal extremities, followed by severe skin induration and later even organ involvement. In a 2007 survey performed by the American College of Radiology, 156 cases of NSF were reported by 27 responding institutions; 140 of these 156 patients were known to have received GBCM. In 78 patients, the specific GBCM was known. Forty-five of them received gadodiamide, 17 gadopentetate dimeglumine, 13 gadoversetamide, and 3 gadobenate dimeglumine. NSF following gadoteridol administration has also been reported. Many of the cases in which agents other than gadodiamide and gadopentetate dimeglumine were used are confounded by the fact that affected patients were injected with other agents as well (ACR, 2008). Patients with chronic kidney disease (CKD) have a 1% to 7% chance of developing NSF after MRI imaging with gadolinium agents (Todd et al, 2007). Patients with GFR less than 30 mL/min/1.73m2 (not on chronic dialysis) are the most difficult patient population in terms of choosing an imaging modality. They are at risk for CIN if exposed to iodinated contrast media for CT imaging and are also at significant risk of developing NSF if exposed to GBCM during MRI. Recent data suggest that the risk of NSF may be greatest in patients with a GFR of less than 15 mL/min/1.73m2 and much less in patients with GFRs that are higher. Patients with severe chronic kidney disease have a 1% to 7% chance of developing NSF after GBCM MRI (Kanal et al, 2008). In the chronic kidney disease patient population, it is recommended that contrast media be avoided if possible. If MRI contrast media is absolutely essential, use of the lowest possible doses (needed to obtain a diagnostic study) of selected GBCM is recommended. In this setting, the patient should be informed of the risks of GBCM administration and must give their consent to proceed. There is no proof that any GBCM is completely safe in this patient group; however, some have suggested avoiding gadodiamide and considering use of macrocyclic agents (Kanal et al, 2008). Patients with CKD but GRF greater than 30 mL/min/1.73m2 are considered to be at extremely low or no risk for developing NSF if a dose of GBCM of 0.1 mmol/kg or less is used. Patients with GFR greater than 60 mL/min/1.73 m2 do not appear to be at increased risk of developing NSF, and the current consensus is that all GBCM can be administered safely to these patients. In their publications, the American College of Radiology stresses that the current information on NSF and its relationship to GBCM administration is preliminary and further research is necessary to better understand this potentially devastating complication. Key Points Contrast Media Upright films may be helpful in certain situations. In the rare case of suspected symptomatic renal ptosis, IVU can be particularly helpful (Fig. 4–3). Supine films are compared with upright films to measure the degree of ptosis. Such a comparison cannot be made with MRI or CT. In the case of calyceal stones or milk of calcium stones, layering of the contrast can be helpful to evaluate the anatomy of the calyx harboring the stones. Although plain film radiography is often used in the evaluation of renal colic, it is unreliable in the demonstration of calculus disease for various reasons: (1) overlying stool and bowel gas may obscure small calculi; (2) stones may be obscured by other structures such as bones or ribs (Fig. 4–4); (3) calcifications in pelvic veins or vascular structures may be confused with ureteral calculi; and (4) stones that are poorly calcified or composed of uric acid may be radiolucent. Nevertheless, plain film radiography is valuable in assessing the suitability of a patient for extracorporeal shock wave lithotripsy because the ability to identify the stone on fluoroscopy is critical to targeting. Furthermore, a KUB film is cost-effective for monitoring residual stone burden after treatment (Fig. 4–5). For complex pathology of the urinary tract, plain abdominal radiography has been supplanted by axial imaging. Plain radiography has a limited role in evaluating soft tissue abnormalities of the urinary tract. The other commonly employed method is the use of an obstructing ureteral catheter such as a bulb-tip, cone-tip, or wedge-tip catheter. These catheters are inserted into the ureteral orifice and then pulled back against the orifice to effectively obstruct the ureter. Contrast is then injected to opacify the ureter and intrarenal collecting system. Depending on the indication for the study, it is useful to dilute the contrast material to 50% or less with sterile fluid. This prevents subtle filling defects in the collecting system or ureter from being obscured. Contrast is injected slowly, usually requiring from 5 to 8 mL to completely opacify the ureter and intrarenal collecting system in adults (Fig. 4–6). More or less contrast may be required depending on the size of the patient and the capaciousness of the collecting system. Limited use of fluoroscopy while injecting will help prevent overdistension of the collecting system and reduce the risk of extravasation of contrast. Care should be taken to evacuate air bubbles from the syringe and catheter before injection. Such air bubble artifacts could be mistaken for stones or tumors. Backflow occurs during retrograde pyelography when contrast is injected under pressure and escapes the collecting system. Contrast may escape the collecting system by one of four routes: Pyelotubular backflow occurs when contrast fills the distal collecting ducts producing opacification of the medullary pyramids (Fig. 4–7A). Pyelosinus backflow occurs when a tear in the calyces at the fornix allows contrast to leak into the renal sinus (Fig. 4–7B). Pyelolymphatic backflow is characterized by opacification of the renal lymphatic channels (Fig. 4–7C). Pyelovenous backflow is seen when contrast enters the venous system, resulting in visualization of the renal vein. Although backflow does not usually cause measurable clinical harm, the potential implications of backflow include (1) introduction of bacteria from infected urine into the vascular system and (2) the absorption of contrast media, which could result in adverse reactions in susceptible patients. It has been demonstrated that the risk of significant urinary tract infection is only about 10% and the risk of sepsis is low when antibiotic prophylaxis therapy is administered before endoscopic procedures (including retrograde pyelography) (Christiano et al, 2000). Although contrast reactions are rare with retrograde pyelography, they have been reported (Johenning, 1980; Weese et al, 1993). In patients with documented severe contrast allergy, prophylactic pretreatment may be appropriate. In those patients considered at risk, care should be taken to inject under low pressures to minimize the probability of backflow and absorption of the contrast into the vasculature system. Loopography is a diagnostic procedure performed in patients who have undergone urinary diversion. Historically the term “loopogram” has been associated with ileal conduit diversion but may be used in reference to any bowel segment serving as a urinary conduit. When imaging patients with a continent diversion involving a reservoir or neobladder, “pouch-o-gram” would be more accurately descriptive. Because an ileal conduit urinary diversion usually has freely refluxing ureterointestinal anastomoses, the ureters and upper collecting systems may be visualized. In other forms of diversion, the ureterointestinal anastomoses may be purposely nonrefluxing. In such circumstances, when opacification of the upper urinary tract is desirable, antegrade ureteral imaging such as IVU, CT, or MRI urography or antegrade nephrostography may be required. When the patient has compromised renal function or is allergic to iodinated contrast material, loopography can be performed with a low risk of systemic absorption (Hudsen et al, 1981). The patient is positioned supine. An abdominal plain radiograph is obtained before the introduction of contrast material (Fig. 4–8A). A commonly employed technique is to insert a small-gauge catheter into the ostomy of the loop, advancing it just proximal to the abdominal wall fascia. The balloon on such a catheter can then be inflated to 5 to 10 mL with sterile water. By gently introducing contrast through the catheter, the loop can be distended, usually producing bilateral reflux into the upper tracts. Oblique films should be obtained in order to evaluate the entire length of the loop (Fig. 4–8B). Because of the angle at which many loops are constructed, a traditional anteroposterior (AP) view will often show a foreshortened loop and could miss a substantial pathology. A drain film should be obtained (Fig. 4–8C). This may demonstrate whether there is obstruction of the conduit. A plain film radiograph is obtained before injection of contrast. The patient is usually positioned slightly obliquely to allow evaluation of the full length of urethra. The penis is placed on slight tension. A small catheter may be inserted into the fossa navicularis with the balloon inflated to 2 mL with sterile water. Contrast is then introduced via catheter-tipped syringe. Alternatively, a penile clamp (e.g., Brodney clamp) may be used to occlude the urethra around the catheter (Fig. 4–9). The patient is positioned supine. A plain radiograph is performed to evaluate for stones and residual contrast and to confirm position and technique. The bladder is filled with 200 to 400 mL of contrast depending on bladder size and patient comfort. Adequate filling is important to demonstrate intravesical pathology or bladder rupture. Oblique films should be obtained because posterior diverticula or fistulae may be obscured by the full bladder. A postdrainage film completes the study (Fig. 4–10). Abdominal and pelvic CT is so commonly used in the evaluation of blunt or penetrating trauma to the abdomen that CT cystography is often performed in conjunction with the trauma evaluation. However, studies have shown that conventional static cystography is as sensitive as CT cystography in detecting bladder rupture (Quagliano et al, 2006; Broghammer and Wessells, 2008). The study may be performed with the patient supine or in a semiupright position using a table capable of bringing the patient into the full upright position. A preliminary pelvic plain radiograph is obtained. In children a 5- to 8-Fr feeding tube is used to fill the bladder to the appropriate volume. Patient comfort should be taken into account when determining the appropriate volume. In the adult population a standard catheter may be placed and the bladder filled to 200 to 400 mL. The catheter is removed and a film is obtained. During voiding, AP and oblique films are obtained. The bladder neck and urethra may be evaluated by fluoroscopy during voiding. Bilateral oblique views may demonstrate low-grade reflux, which cannot be appreciated on the AP film. In addition, oblique films will demonstrate bladder or urethral diverticula, which are not always visible in the straight AP projection. Postvoiding films should be performed (Fig. 4–11). This study requires bladder filling using a catheter. This may be traumatic in children and difficult in some patients with anatomic abnormalities of the urethra or bladder neck. Filling of the bladder may stimulate bladder spasms at low volumes, and some patients are unable to hold adequate volumes for investigation. Bladder filling in patients with spinal cord injuries higher than T6 may precipitate autonomic dysreflexia (Barbaric, 1976; Fleischman and Shah, 1977; Linsenmeyer et al, 1996). All ultrasound imaging is the result of the interaction of sound waves with tissues and structures within the human body. Ultrasound waves are produced by applying short bursts of alternating electrical current to a series of crystals housed in the transducer. Alternating expansion and contraction of the crystals via the piezoelectric effect creates a mechanical wave that is transmitted through a coupling medium to the skin and then into the body. The waves that are produced are longitudinal waves. In a longitudinal wave the particle motion is in the same direction as the propagation of the wave (Fig. 4–12). This motion produces areas of rarefaction and compression of tissue in the direction of travel of the ultrasound wave (Fig. 4–13). A portion of the wave is reflected toward the transducer. The transducer then serves as a receiver and “listens” for the returning sound wave reconverting the mechanical to electrical energy. The transducer must be in direct, secure contact with the subject to transmit and receive the reflected sound waves. (Adapted from Merritt CRB. Physics of ultrasound. In: Rumack CM, Wilson SR, Charboneau JW, editors: Diagnostic ultrasound. 3rd ed. St Louis: Elsevier; 2005. p. 4.)
Conventional Radiography
Physics
Radiation Management in Uroradiology
Relative Radiation Levels
Radiation Protection
Contrast Media
Intravascular Iodinated Contrast Media
Adverse Reactions to Intravascular Iodinated Contrast Media
Contrast Complications
Severe Reactions
Premedication Strategies
Prednisone
50 mg by mouth at 13 hr, 7 hr, and 1 hr before contrast media injection
Plus diphenhydramine (Benadryl)—50 mg IV, IM, or by mouth 1 hr before contrast medium injection
Methylprednisolone (Medrol)
32 mg by mouth 12 hr and 2 hr before contrast media injection
Plus diphenhydramine (Benadryl)—50 mg IV, IM, or by mouth 1 hr before contrast medium injection
Specific Clinical Considerations
Metformin
Contrast-Induced Nephropathy
Magnetic Resonance Imaging Contrast Agents
Nephrogenic Systemic Fibrosis
Intravenous Urography
Technique
Plain Abdominal Radiography
Limitations
Retrograde Pyelography
Technique
Complications
Loopography
Technique
Retrograde Urethrography
Technique
Static Cystography
Technique
Limitations
Voiding Cystourethrogram
Technique
Limitations
Ultrasonography
Principles
Urinary Tract Imaging: Basic Principles
• The effective radiation dose describes the potential for adverse health effects from ionizing radiation.
• The effective dose is a quantity used to denote the radiation risk (expressed in sieverts) to a population of patients from an imaging study. See Table 4–1 for a description of the relationship between these measures of radiation exposure.
• Type II diabetics with renal insufficiency on oral metformin biguanide hyperglycemic therapy are at risk for developing biguanide lactic acidosis after exposure to intravascular radiologic contrast media and should stop metformin the day before the procedure and restart 48 hours after if they have a normal or baseline serum creatinine.