Eric S. Rovner, MD
General Considerations
Acquired urinary fistulae in the industrialized world are almost universally unexpected and may result in a great deal of inconvenience, discomfort, and physical disability for the affected individual. They are most often acquired as a result of a medical or surgical intervention for an unrelated problem, and, consequently, considerable emotional and psychologic distress often accompanies the diagnosis and subsequent treatment. This may be expressed as anger, resentment, and disappointment on the part of the patient toward the physician. As a result, not infrequently, the medicolegal aspects of these cases can be very disturbing to the treating health care practitioner, with an increasing proportion of these cases being adjudicated in court (Thomas and Williams, 2000). Only the most naive surgeon would fail to recognize that many of these fascinating reconstructive cases often have significant medicolegal implications, especially in the setting of iatrogenic fistula creation. Nevertheless, minimizing patient discomfort, maintaining a positive and honest patient–physician relationship while providing constant reassurance, and, finally and perhaps most importantly, pursuing expeditious and successful treatment of the fistula will most often result in a satisfactory, non-confrontational, mutually satisfying long-term outcome.
The principles of repair of urinary fistulae are outlined in Table 77–1 and can be applied to virtually any type of fistula involving the urinary tract (see Table 77–1). Prevention of urinary fistulae is, of course, paramount; however, nutrition, infection, and malignancy are important considerations not only when assessing a patient for the risk of creation of a fistula during any given intervention, but also during an evaluation for the repair of an existing urinary fistula. Although the vast majority of urinary fistulae in the industrialized world occur in healthy, well-nourished individuals, a nutritional assessment may be an important factor in some patients with fistulae, such as those patients with malignancies. Ensuring adequate nutrition is integral to surgical healing in general, but is especially important in the setting of a urinary fistula. Not uncommonly, the catabolic processes contributing to the lack of healing, which may have been a contributing factor in the initial fistula formation, are often ongoing. This is especially relevant in fistulae related to radiation therapy or in debilitated patients.
Table 77–1 Principles of Urinary Fistula Management
Although some types of urinary fistulae will heal with conservative management, surgery often assumes a role in the definitive repair. Repair and reconstruction of urinary fistulae are sometimes complex. These should be approached on a case by case basis, because repair may involve some innovative and even improvisational maneuvers in the operating room. The surgeon should be familiar with a variety of approaches and techniques, because one approach will not be optimal for all patients with a given type of urinary fistulae. Principles of surgical management of urinary fistulae are outlined in Table 77–2. The finding of a persistent fistula after presumably definitive treatment may suggest the existence of other contributing host factors, such as malignancy, nutritional issues, the possibility of an unrecognized foreign body, tissue ischemia, or surgical factors, such as inadequate postoperative urinary drainage, persistent distal urinary obstruction, or technical problems with the surgery.
Table 77–2 Principles of Surgical Repair of Urinary Fistula
Urogynecologic Fistulae
Vesicovaginal Fistulae
Vesicovaginal fistulae (VVF) are the most common acquired fistula of the urinary tract (Gerber and Schoenberg, 1993) and have been known since ancient times (Fig. 77–1). However, it was not until 1663 that Hendrik von Roonhuyse first described surgical repair of VVF by denuding the fistula margins and then reapproximating them with sharpened stiff swan quills (Margolis and Mercer, 1994). Johann Fatio is generally credited with the first successful VVF repair, in 1675, using von Roonhuyse’s technique (Falk and Tancer, 1954). In 1838, using leaden suture, John Peter Mettauer was the first U.S. surgeon to claim a successful VVF closure (Kight, 1967). In 1852, James Marion Sims published his now famous surgical series describing his method of surgical treatment of VVF using silver wire in a transvaginal approach (Sims, 1852). Of note, it was not until his 30th attempt at closure of VVF that he achieved success. However, Sims remains the subject of considerable debate regarding his ethics (Richardson, 1994; Sartin, 2004), because it is unknown whether the patients in his surgical series were willing and consenting participants (all were African-American slaves in pre–Civil War America). He was later to become one of the great figures in the history of operative gynecology. The first successful transabdominal approach to VVF repair was reported by Trendelenburg in 1888, and the concept of an interpositional flap was first proposed and reported in 1928 by Martius, who used a labial fat pad.
Etiology and Prevalence
The etiology of VVF differs in various parts of the world. In the industrialized world, the most common cause (>75%) of VVF is injury to the bladder at the time of gynecologic, urologic, or other pelvic surgery (Symmonds, 1984; Lee et al, 1988; Tancer, 1992). Surgical injury to the lower urinary tract most commonly occurs in the setting of hysterectomy (Fig. 77–2), whereas most of the remainder are related to general surgery procedures in the pelvis, anterior colporrhaphy or cystocele repair, anti-incontinence surgery, or other urologic procedures (Armenakas et al, 2004). Of 207 VVF repaired at the University of Caliornia–Los Angeles (UCLA) over a 10-year period ending in 2001, Eilber and colleagues (2003) reported the cause as abdominal hysterectomy in 83%, vaginal hysterectomy in 8%, radiation in 4%, and miscellaneous in 5%. In 1964, Massee and colleagues from the Mayo Clinic reviewed the cause of urogenital fistulae in 262 patients and cited uterine operations as a proximate cause in 73.7%, vaginal wall operations in 6.5%, urinary tract operations in 6.9%, and obstetric operations in 6.5%, with the remainder being due to miscellaneous causes. A later series of greater than 300 fistulae from the same institution cited gynecologic surgery as the cause of VVF in 82%, obstetric procedures in 8%, radiation in 6%, and trauma or fulguration in 4% (Lee et al, 1988). Other causes of VVF in the industrialized world include malignancy, pelvic radiation, and obstetrical trauma (including forceps lacerations and uterine rupture) (Everett and Mattingly, 1956; Gerber and Schoenberg, 1993). The use of vaginal mesh for prolapse repair may also result in VVF (Margulies et al, 2008; Ridgeway et al, 2008). Although fortunately uncommon during labor, approximately 22% of uterine ruptures are associated with a bladder injury (Raghavaiah and Devi, 1975). Prior to 1900, the most common cause of VVF in the United States was obstructed labor (Stothers et al, 1996). However, obstructed labor and obstetric trauma, in general, now account for very few VVF in the United States and other industrialized nations, probably due to the widespread availability of excellent prenatal and perinatal obstetric care.
The rate of iatrogenic bladder injury during abdominal hysterectomy is estimated to be between 0.5% and 1.0% (Keettel et al, 1978). Mathevet and colleagues (2001) reported the incidence of bladder injury during vaginal hysterectomy to be 1.7% in 3076 cases, with all injuries being recognized and repaired intraoperatively. Despite the immediate intraoperative repair reported in this series, there were four VVF noted, giving a crude VVF rate during vaginal hysterectomy of 0.13%. The reported incidence of intraoperative bladder injury varies considerably in the literature, depending on whether routine cystoscopy was performed. In series where cystoscopy was not performed, the overall rate of bladder injury was reported to be approximately 2.6 per 1000 cases, whereas in series where cystoscopy was routinely performed, the overall rate of bladder injury was approximately 10.4 per 1000 cases (Gilmour et al, 1999). The incidence of fistula after hysterectomy is estimated to be approximately 0.1% to 0.2% (Harris, 1995). There are approximately 140 to 150 VVF repairs annually in England and Wales (Hilton, 1997).
Posthysterectomy VVF are thought to result most commonly from an incidental unrecognized iatrogenic cystotomy near the vaginal cuff (Kursh et al, 1988). If unrecognized intraoperatively, a pelvic urinoma may develop and ultimately drain out through the vaginal cuff. Ongoing urinary drainage along this tract results in a fistula. Other potential mechanisms for posthysterectomy VVF include tissue necrosis from cautery, a suture placed through both the bladder and vaginal wall during closure of the vaginal cuff, or an attempt to control pelvic bleeding by suture ligature. Tissue ischemia and then necrosis promotes fibrosis and induration, finally resulting in an epithelial or mucosal lining of the tract and the development of a fistula tract. Possibly, factors other than an isolated suture placed through the bladder and vagina are necessary for posthysterectomy VVF formation because, at least, in an animal model, deliberate suture fixation of the bladder to the vagina does not invariably result in VVF in the absence of infection, urinary extravasation, or other complicating factors (Meeks et al, 1997).
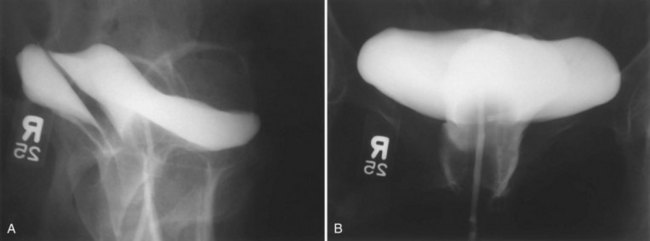
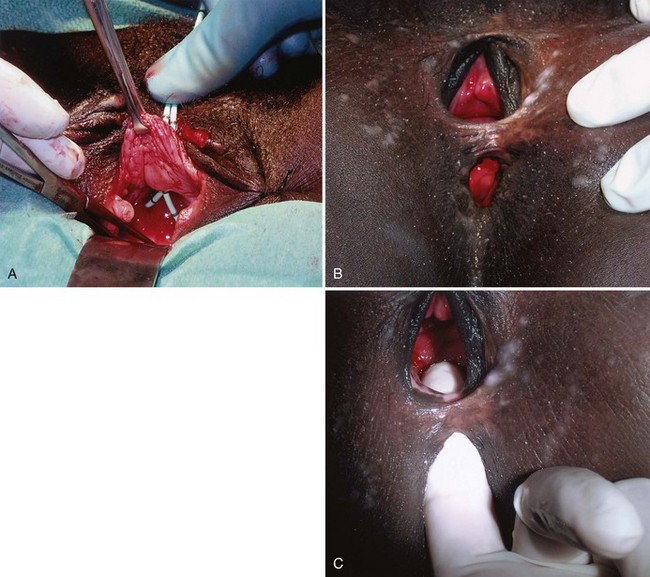
In the developing world where routine perinatal obstetrical care may be limited, VVF most commonly occur as a result of prolonged obstructed labor due to cephalopelvic disproportion, with resulting pressure necrosis to the anterior vaginal wall, bladder, bladder neck, and proximal urethra from the baby (Arrowsmith et al, 1996) (Fig. 77–3). The constellation of problems resulting from obstructed labor is not limited to VVF and has been termed the “obstructed labor injury complex” and includes varying degrees of each of the following: urethral loss, stress incontinence, hydroureteronephrosis, renal failure, rectovaginal fistula, rectal atresia, anal sphincter incompetence, cervical destruction, amenorrhea, pelvic inflammatory disease, secondary infertility, vaginal stenosis, osteitis pubis, and foot drop (Arrowsmith et al, 1996). The obstructed labor injury complex occurs largely in developing countries in certain cultures due to several factors, including (1) marriage and conception at a very young age, which results in childbearing in a relatively small and immature pelvis, (2) poor nutrition resulting in stunted skeletal (e.g., pelvic) growth in the mother, and (3) relative absence of qualified prenatal and obstetrical care (Margolis and Mercer, 1994). Patients may suffer with obstructed labor for days in a rural environment, eventually traveling to a distant health care facility only to have a stillborn fetus and a VVF. In direct contradistinction to the epidemiology of VVF in the industrialized world, 96.5% of 932 VVF seen at a single hospital in Nigeria over a 7-year period were temporally associated with labor and delivery (Wall et al, 2004). The incidence of obstetric fistula in developing countries has been estimated at approximately 0.3% to 0.4% of deliveries (Margolis et al, 1994), or between 1 and 4 per 1000 vaginal deliveries (Margolis et al, 1994; Danso et al, 1996). In sub-Saharan Africa the incidence rate has been estimated at 10.3 per 100,000 deliveries (Vangeenderhuysen et al, 2001). An estimate of up to 500,000 new cases of obstetric fistula occur throughout the world annually (Hilton, 2003), although the total morbidity from obstructed maternal labor has been estimated to be in excess of 5 million individuals annually (Kelly, 1991). Risk factors include young or old maternal age, and primigravids (Danso et al, 1996).
(A to C, Courtesy of Mark Morgan, MD, Department of Obstetrics and Gynecology, Hospital of the University of Pennsylvania, Philadelphia, PA.)
Obstetric fistulae tend to be larger, located distally in the vagina, and may involve large portions of the bladder neck and proximal urethra. Even in experienced hands, these are often very difficult to repair due to the extensive soft tissue loss, as well as the ischemia and fibrosis of adjacent tissues (Arrowsmith, 1994; Elkins, 1994). Obstetric fistulae are devastating injuries in the developing world, with significant socioeconomic ramifications (Donnay and Weil, 2004; Wall et al, 2004). The affected individuals, commonly young teens, are often ostracized and shunned from family and friends and become permanent outcasts in society. Only rarely are these individuals able to get adequate care and repair due to the lack of organized health care in many countries in the developing world. Major efforts are underway to improve education, the socioeconomic status of women, and access to health care in these regions (Tahzib, 1983; Tahzib, 1989; Kelly, 1991; Wall, 1996; Donnay and Weil, 2004; Kelly, 2004). With improvements in the delivery of prenatal care in some areas of the developing world, it appears that the incidence of VVF due to obstetric causes may be decreasing as a subset of all VVF, with a proportional increase in the rate of iatrogenic gynecologic fistulae approaching that in industrialized nations (Ayhan et al, 1995; Obi et al, 2008). Another important cause of VVF in some parts of the world is traditional folk treatments such as “gishiri cutting,” which may account for up to 13% of VVF in some series (Tahzib, 1983). This ritual practice involves using a knife to incise the anterior vagina as a treatment for a variety of conditions, including infertility, dyspareunia, dysuria, and back pain.
Other causes of VVF include urologic or gynecologic instrumentation, including percutaneous procedures (Ramsay et al, 1992; Pruthi et al, 2000), retroperitoneal, vascular or pelvic surgery, infectious and inflammatory diseases (Borjas and Rodriguez Diaz 1949; Bland and Gelfand, 1970; Ba-Thike et al, 1992; Monteiro et al, 1995), foreign bodies (including neglected pessaries) (Binstock et al, 1990; Goldstein et al, 1990; Grody et al, 1999), congenital VVF (Rousseau et al, 1996), sexual trauma (Roy et al, 2002), vaginal laser procedures (Colombel et al, 1995), and external violence (see Table 77–3). The three most common locally advanced malignancies that result in VVF include cervical, vaginal, and endometrial carcinoma, which in aggregate account for approximately 3% to 5% of VVF in the industrialized world.
Table 77–3 Etiology of Vesicovaginal Fistula
VVF due to radiation therapy deserve special mention. These VVF may occur several decades after completion of the radiation (Zoubek et al, 1989). The incidence of urinary fistula following radiation therapy varies with the type, dose, and location of the radiation. Both external beam and interstitial (Aristizabal et al, 1983) radiation therapy may result in VVF. An incidence of 1.6% of any type of urinary fistula was noted in one series of greater than 2200 patients treated with a variety of different radiation modalities for cervical carcinoma (Alert et al, 1980). Perez reported a 0.6% to 2.0% incidence of VVF formation in 1456 patients undergoing combined external beam radiotherapy and brachytherapy for stages I to III cervical cancer (Perez et al, 1999). In a series of 2096 patients undergoing therapy for cervical cancer, there was an overall genital fistula rate of 1.8%, including rectovaginal fistulae. All patients diagnosed with fistula had received radiation therapy, and the median interval from completion of radiation to presentation of VVF was 8.7 months (Emmert and Kohler, 1996). Higher radiation doses seem to correlate with a greater risk for overall morbidity, as well as fistula formation (Perez et al, 1984; Perez et al, 1999). The endarteritis as a result of the radiation therapy may involve the surrounding tissues, limiting reconstructive options. An important consideration in any fistula following radiation therapy for malignancy is the possibility that the fistula represents a recurrence of the malignancy. Therefore biopsy of fistula tract should be strongly considered prior to considering definitive repair in these patients.
Intraoperative Risk Factors for Iatrogenic VVF
Clearly, intraoperative injury to the urinary bladder is a primary risk factor for subsequent development of a postoperative VVF. Other risk factors for postoperative VVF formation include prior uterine surgery (cesarean section), endometriosis, infection, diabetes, arteriosclerosis, pelvic inflammatory disease, and prior radiation therapy (Blandy et al, 1991; Likic et al, 2008). In a large series of VVF, Tancer and colleagues (1992) described several factors that increased the risk for VVF following hysterectomy, including prior uterine operation (especially cesarean section), endometriosis, recent cold-knife cervical conization, and prior radiation therapy. Nevertheless, almost 30% of the patients in this series had no identifiable risk factor. The operative approach to hysterectomy is an important factor, because bladder injuries are at least three times more common during abdominal hysterectomy compared with vaginal hysterectomy. Intraoperative recognition and repair of bladder injury is paramount in prevention of VVF; however, despite intraoperative repair, VVF may still result in a substantial number of patients. In 1969, Hutch emphasized five factors in the prevention of VVF during gynecologic surgery: (1) immediate detection of bladder injury using vital dyes if necessary; (2) watertight closure of the bladder; (3) satisfactory extravesical drain placement; (4) avoidance of a vaginal incision, if possible, following recognition of the bladder injury; and (5) prolonged, uninterrupted postoperative bladder drainage (Hutch and Noll, 1970).
Clinical Features
Evaluation and Diagnosis
Presentation
VVF following hysterectomy or other surgical procedures may present upon removal of the urethral catheter, or VVF may present 1 to 3 weeks later with urinary drainage per vagina. It may be possible to identify some patients at high risk for VVF in the immediate postoperative period. Kursh and colleagues (1988) noted that patients who developed VVF following hysterectomy more commonly had postoperative ileus, hematuria, bladder irritability, and elevated white blood cell count compared with a cohort of patients who did not develop VVF. Occasionally, posthysterectomy VVF may go undiagnosed for an extended period of time, because postoperative clear or serosanguinous vaginal discharge is often attributed to the surgery itself.
Physical Examination
A pelvic exam with a speculum should always be performed in the evaluation of VVF. The bivalved speculum examination usually provides a precise assessment of VVF including the location, size, and number of fistulae. Vaginoscopy has been suggested as an adjunct measure, in some cases, using a modified endoscope to precisely visualize the fistula tract (Redman, 1990). Most commonly, VVF following hysterectomy are located along the anterior vaginal wall at the level of the vaginal cuff (Fig. 77–4). A visual and manual assessment of inflammation surrounding the fistula is necessary, because it may affect timing of the repair. Significant inflammation, infection, or induration around the fistula may mitigate against immediate repair. Relevant vaginal anatomy, including depth, associated prolapse, atrophy, and introital size are carefully recorded, because these may affect the surgical approach to repair. Anatomically, fistulae located high in the vagina, at the level of the hysterectomy cuff in a deep narrow vagina, may be best approached by some surgeons abdominally because a vaginal approach in these patients can be challenging. This may be especially relevant in nulliparous females in whom there is usually very limited pelvic floor laxity or vaginal prolapse. Postmenopausal vaginal atrophy may be treated with preoperative topical estrogen replacement, thereby optimizing the health and vascularity of potential reconstructive flaps. Palpation for masses or other pelvic pathology, which may require attention at the time of fistula repair, is also performed. Notation of prior incisions in the perineum, lower abdomen, and thigh is necessary because these tissues may be required for flap reconstruction when definitive repair is undertaken.
The presence of a VVF may be confirmed by instilling a vital blue dye into the bladder per urethra and observing whether vaginal drainage is discolored. Small or occult fistulae may be identified in this fashion (Drutz and Mainprize, 1988). A colored solution, such as methylene blue or indigo carmine, is mixed into solution and infused into the bladder. The vagina may be packed with gauze or directly inspected for blue-tinged leakage. If blue-tinged leakage is not apparent and the diagnosis of VVF is in doubt, the sensitivity of this test may be improved by placing a vaginal packing and ambulating the patient for a short period of time. Staining at the introital (distal) end of the packing suggests urinary incontinence or a urethrovaginal fistula, whereas proximal staining suggests a VVF. If the vaginal packing remains dye-free with this maneuver, then the possibility of a ureterovaginal fistula can be investigated with the use of a clean vaginal packing, intravenous indigo carmine (or other vital dye), and a repeat pad test. Blue staining at the proximal end of the pad, following this maneuver, suggests the presence of a ureterovaginal fistula.
A double dye or tampon test may confirm the diagnosis of urinary fistula, as well as suggest the possibility of an associated ureterovaginal or urethrovaginal fistula (Moir, 1973; Raghavaiah, 1974). In one variation of the double dye test, a tampon is placed per vagina. Oral phenazopyridine is administered, and vital blue dye is instilled into the bladder. If the tampon is discolored yellow-orange at the top, it is suggestive of a ureterovaginal fistula. Blue discoloration in the midportion of the tampon suggests VVF, whereas blue staining at the bottom suggests a urethrovaginal fistula.
Clear vaginal discharge following hysterectomy does not invariably represent a urinary fistula or incontinence. Other than normal vaginal secretions, less common causes include a peritoneovaginal fistula (Ginsberg, 1998), lymphatic fistula (Lau and Wong, 1994), vaginitis, and fallopian tube fluid (Leach, 1987). Incontinence following caesarian section, in which a VVF has been excluded, may suggest the possibility of a ureterovaginal or vesicouterine fistula.
Cystoscopy
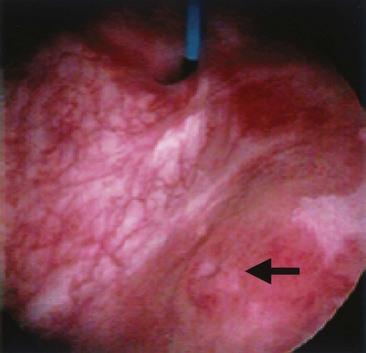
An endoscopic examination should be performed in patients with a suspicion of VVF (Fig. 77–5). Immature fistulae may appear as an area of localized bullous edema without distinct ostia. Mature fistulae may have smooth margins with variably sized ostia. In some cases, multiple pits and cavities along an area of the traumatized posterior bladder wall, in the setting of a small VVF, may make it difficult to identify the exact fistula tract. In these cases, a guidewire or ureteral catheter may be placed through the working channel of the cystoscope and into the fistula tract (Fig. 77–6). Visualization of the wire in the vagina confirms the exact location of the VVF on both the bladder and genital sides. Cystourethroscopy can confirm the presence of the fistula, but also may assess the size of the tract, the presence of collateral fistulae, and the location of the ureteral orifices in relation to the fistula. Small fistulae, usually less than 3 to 4 mm in diameter may be amenable to simple fulguration, which can be performed at the time of cystoscopy (see later discussion) (Stovsky et al, 1994). Importantly, in the setting of a prior history of pelvic malignancy, a biopsy of the fistula is often done to evaluate for the possibility of a recurrent malignancy. Fistulae located near or at the ureteral orifice may require ureteral reimplantation at the time of VVF repair. This type of requirement would usually mitigate against a completely transvaginal attempt at repair.
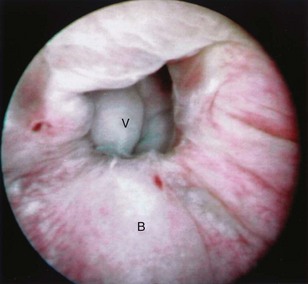
Figure 77–5 Endoscopic view of vesicovaginal fistula (VVF). This is the same patient as in Figure 77–4. The fistula is now seen from the bladder side. This VVF is large enough to see directly into the vagina (V) through the bladder (B).
Imaging
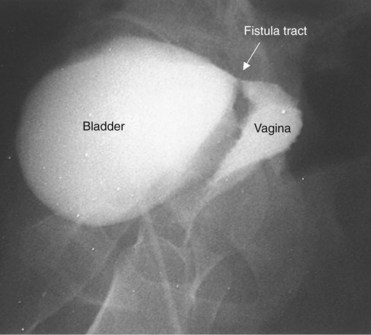
A cystogram and/or voiding cystourethrogram (VCUG) and an upper tract study should be performed in patients being evaluated for a VVF. The cystogram may objectively determine the presence and location of the fistula. Upon filling of the bladder, contrast often begins to opacify the vagina, almost immediately confirming the presence of a VVF. VVF are often best seen in the lateral projection (Fig. 77–7) in which the bladder and vagina are not superimposed. Often, the actual VVF tract may be visible in the lateral projection (Fig. 77–8). However, voiding images may be necessary in some patients with small fistulae, to demonstrate the VVF. The slight increase in intravesical pressure that accompanies micturition is usually adequate to demonstrate even very small fistulae. Importantly, a cystogram that fails to demonstrate a suspected VVF, but lacks voiding images or postvoid images, should be considered nondiagnostic. During voiding, care should be taken to exclude vaginal voiding or reflux of contrast from the introital region cephalad into the vagina, which would produce a falsely positive image. An involuntary bladder contraction can be provoked with rapid filling during cystography, and if the intravesical pressure rises sufficiently, this may also be sufficient to demonstrate a VVF when the filling images of the cystogram failed to demonstrate it. In some instances, a cystogram can also make an assessment of bladder capacity (important in the setting of prior radiotherapy), cystocele, bladder neck competence, and vesicoureteral reflux, any of which may have an impact on operative repair.
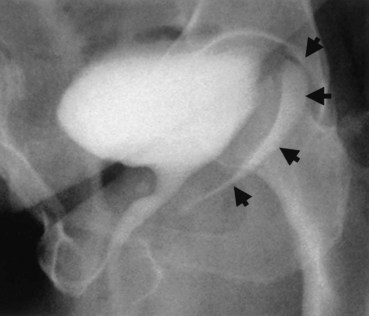
Up to 12% of postsurgical VVF have an associated ureteral injury or ureterovaginal fistula (Goodwin and Scardino, 1980); thus, upper urinary tract evaluation is important. Intravenous urography (IVU) (Gerber and Schoenberg, 1993), or, more recently, computed tomography (CT) urography is usually sufficient for this purpose. In one series of 216 consecutive patients with VVF due to obstructed labor, almost 50% of patients were diagnosed with an upper tract abnormality on IVU; caliectasis was found in 71% of those affected; however, almost 10% were found to have a nonfunctioning renal unit (Lagundoye et al, 1976). If there is a suspicion for a ureterovaginal fistula, or if the distal ureter is not well seen IVU, retrograde pyelography may be performed (Blandy et al, 1991) (Fig. 77–9).
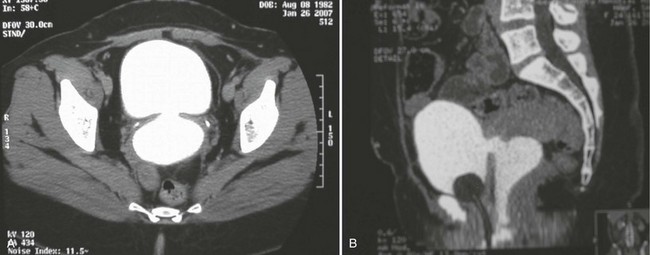
In addition to contrast cystography and VCUG, CT, ultrasonography, and magnetic resonance imaging (MRI) have been used in the evaluation of VVF (Kuhlman and Fishman, 1990; Outwater and Schiebler, 1993; Yang et al, 1994). Delayed CT visualization of contrast within the vagina is considered highly suspicious for VVF in the majority of cases (Kuhlman and Fishman, 1990) (Fig. 77–10). In cases of suspected VVF, CT should be performed with only intravenous contrast, or, alternatively, a CT cystogram can be performed to isolate the bladder. A vaginal tampon placed per vagina during IVU or CT scan may improve the sensitivity for finding small or occult VVF in patients with an otherwise negative evaluation (Wesolowski and Meaney, 1977). Cross-sectional imaging may also be helpful in assessing for recurrent malignant disease in those with such a history.
Other Studies
Appropriate urine studies including culture, and cytology when indicated, are performed. When infection is detected, appropriate antibiotic coverage is initiated. Urodynamics, including videourodynamics, are generally not necessary in the evaluation of routine posthysterectomy VVF. However, in the setting of a prior history of radiation, radical pelvic surgery (e.g, radical hysterectomy), or in those with preexisting neurogenic vesicourethral dysfunction, a urodynamic evaluation can be used to evaluate for significant detrusor dysfunction, including impaired bladder compliance. In patients with a history of significant symptoms of voiding dysfunction and incontinence preceding the VVF, urodynamic evaluation may help to define these symptoms prior to repair of the VVF (Hilton, 1998), an important element in preoperative counseling regarding potential outcomes and preparation for the optimal operative plan.
Treatment
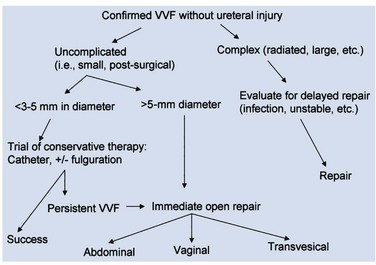
Conservative and Minimally Invasive Therapy
Regardless of the aforementioned limitations and drawbacks, a trial of indwelling catheterization and anticholinergic medication for at least 2 to 3 weeks may be warranted in selected patients with newly diagnosed VVF, because spontaneous healing may result (Davits and Miranda, 1991). This is especially applicable in those patients in whom the initial placement of the catheter immediately resolves the vaginal leakage. Tancer (1992) reported three patients with immature VVF tracts who were successfully treated expectantly with indwelling catheterization. All three patients were seen within 3 weeks of the initial surgery and were found to have nonepithelialized VVF tracts on physical examination. Fistulous tracts that remain open 3 or more weeks after adequate Foley drainage are unlikely to resolve without further intervention, especially those which appear completely epithelialized on examination.
Patients with small epithelialized fistulae may benefit from a minimally invasive treatment involving disruption of the epithelial layer of the fistula tract (Fig. 77–11). Catheterization may be combined with minimally invasive electrocoagulation of the fistula tract. In this approach, a small cautery electrode is passed into the fistula tract endoscopically as far as possible. The electrode is slowly withdrawn from the tract with the electrode set on coagulation. The edges of the fistula tract should blanche. Care is taken not to overcoagulate, because this can cause widespread tissue necrosis, sloughing, and enlargement of the fistula. This approach was advocated by O’Conor, as far back as 1938, for small, highly-situated fistulae (O’Conor and Sokol, 1951). Stovsky and colleagues (1994) demonstrated success with endoscopic electrocoagulation and bladder drainage in 11 of 15 cases. All patients had VVF less than 3.5 mm in diameter and were drained for a minimum of 2 weeks post-treatment. It was suggested that disruption of the epithelial component of the VVF tract with subsequent fibrosis, scarring, and closure of the tract is the mechanism by which electrocoagulation exerted its favorable effects. Falk and Orkin (1957) successfully treated eight patients with electrocoagulation and catheter drainage for 10 days. All successfully treated patients had VVF less than 3 mm in diameter; two patients with 6-mm VVF failed this approach. Importantly, in patients with a thin vesicovaginal septum, large VVF, a nonoblique fistula tract, or those with significant inflammation around the fistula tract, fulguration risks failure and the possibility of enlarging the size of the fistula. This approach may also devitalize adjacent tissues, thereby compromising their future utility as flaps. Fibrin sealant has been used as an adjunctive measure to treat VVF (Pettersson et al, 1979; Hedelin et al, 1982). The fibrin sealant may be injected directly into the fistula tract following fulguration as described above. The bladder is then drained for several weeks. Presumably, the gel-like nature of the fibrin sealant plugs the hole until tissue ingrowth occurs from the edges of the fistula. Fibrin sealant has been used successfully in combination with (Morita and Tokue, 1999) and without (Evans et al, 2003b) bovine collagen as an additional “plug.” In general, these conservative measures are useful for small, oblique fistulae (usually less than 2 to 3 mm in diameter), in patients who are agreeable to this course of therapy.
Case reports of other minimally invasive VVF repair techniques have been reported, including laser tissue welding with a Nd-YAG laser (Dogra and Nabi, 2001), as well as transurethral endoscopic suturing (Okamura et al, 1997; McKay, 2001).
Surgical Repair
It has been stated that the best opportunity to achieve successful repair of VVF is with the initial operation (Weed, 1978; Elkins 1994). Previous failed attempts at repair produce scarring, anatomical distortion, and may compromise potential reconstructive flaps. Therefore careful preoperative planning is essential to maximize the chances for a successful result. There is no “best” approach for all patients with VVF.
Timing: Immediate versus Delayed Repair
The timing of VVF repair is somewhat controversial. Repair of VVF should be as expeditious as possible to minimize patient suffering; however, optimal timing for repair should allow consideration of certain medical and surgical factors as well. It is generally accepted that VVF resulting from obstructed labor should be associated with a 3- to 6-month delay prior to definitive repair (Wein et al, 1980a, Arrowsmith, 1994; Waaldijk, 1994) to allow maximum demarcation of ischemic tissue and resolution of the associated edema and inflammatory reaction. Longer periods of time, up to 6 to 12 months, have been advocated for radiation-induced fistulae (Wein et al, 1980a), which are often associated with severe obliterative endarteritis and reduced tissue vascularity.
In the classical teaching of VVF, a minimal waiting period of several months is suggested—from the inciting event prior to the definitive repair attempt—to allow reduced tissue edema and inflammation, and optimal pliability of the tissues (Persky and Rabin 1973; Lawson, 1978; O’Conor, 1980; Wein et al, 1980a). O’Conor recommended a waiting period of 3 to 6 months for suprapubic VVF repairs (O’Conor et al, 1973). In this setting, reduced inflammation and edema permit easier identification of tissue planes (and therefore flap development), less bleeding, and less tension on the reapproximated suture lines. However, over the ensuing decades, the enthusiasm for delayed management has waned and, in general, uncomplicated postgynecological urinary fistulae may be repaired as soon as they are identified and confirmed, thereby minimizing patient discomfort and anguish (Collins et al, 1971; Persky et al, 1979; Fourie, 1983; Badenoch et al, 1987a; Cruikshank, 1988; Wang and Hadley 1990; Blandy et al, 1991; Blaivas et al, 1995; Kostakopoulos et al, 1998b). This is especially true when they occur as a result of clean surgical trauma (Wang and Hadley, 1990). Nondelayed closure has also been applied to obstetric fistulae with good results (Waaldijk, 1994; Waaldijk, 2004). Nevertheless, in some cases, the timing of a VVF repair is best tailored to the individual patient (Blaivas et al, 1995). Raz and colleagues (1993) suggested that uncomplicated VVF following abdominal hysterectomy could and should be repaired as expediently as possible transvaginally; however, a 2- to 3-month waiting period may be warranted for some VVF following vaginal hysterectomy. Conversely, if an abdominal approach is being considered following a particularly difficult or complicated abdominal surgery that resulted in the VVF (i.e, complicated by abscess, urinoma), then a delay may be warranted to allow resolution of active inflammation. Another potential reason to delay repair is to treat ongoing infection or inflammation at the level of the vaginal cuff. Periodic reexamination of the vaginal tissues can be performed every 1 to 2 weeks and definitive repair scheduled when suitable pliability is noted (Carr and Webster, 1996).
A vaginal approach can be attempted as soon as 2 to 3 weeks after the initial injury, if conservative therapy fails (i.e., the patient remains wet when a Foley catheter is in place to provide adequate drainage of the bladder) and the patient is in good general health. The vaginal tissues are usually relatively undisturbed from the prior causative surgery, especially if the surgery was transabdominal. Wide healthy vaginal flaps can usually be obtained. In the rare circumstance when a VVF presents within the first 24 to 48 hours postoperatively, an immediate repair can be attempted (Margolis and Mercer, 1994); however, it is possible that these VVF, especially if small in diameter, may heal spontaneously with catheterization over the course of several weeks. It is well documented that similar outcomes can be achieved with early versus delayed abdominal and vaginal repair (Collins et al, 1960; Persky et al, 1979; Zimmern et al, 1985; Badenoch et al, 1987a; Wang and Hadley, 1990; Blandy et al, 1991) with success rates in excess of 90% for uncomplicated VVF.
Approach: Abdominal versus Vaginal
Vesicovaginal fistulae may be repaired through a transvaginal or transabdominal (transvesical) approach. Each approach has merits depending on the particular circumstances of the fistula, and excellent outcomes can be expected with both approaches (Table 77–4). Although factors such as size, location, and the need for adjunctive procedures often influence the choice of approach, the most important factor is the experience of the operating surgeon. Thus there is no preferred approach for all fistulae, and the “optimal” approach to the uncomplicated postgynecological VVF is usually the one that is most successful in the individual surgeon’s hands (Gerber and Schoenberg, 1993; Akman et al, 1999). Although it has been a long-held belief that gynecologists prefer to fix VVF transvaginally, and urologists prefer a transabdominal approach due to their respective training and experience, (Edwards, 1982; Gerber and Schoenberg, 1993), this difference is becoming increasingly blurred as urologists gain more experience and comfort operating transvaginally for a variety of disparate indications.
Table 77–4 Abdominal versus Transvaginal Repair of Vesicovaginal Fistula
| ABDOMINAL | TRANSVAGINAL | |
|---|---|---|
| Incision | Abdominal incision | Vaginal incision(s) can be done immediately in the absence of infection or other complications |
| Timing of repair (elapsed time from fistula creation) | Often delayed 3-6 months | |
| Exposure | Fistula located low on the trigone or near the bladder neck may be difficult to expose transabdominally | Fistula located high at the vaginal cuff may be difficult to expose transvaginally |
| Location of ureters relative to fistula tract | Fistula located near ureteral orifice may necessitate reimplantation | Reimplantation may not be necessary even if fistula tract located near ureteral orifice |
| Sexual function | No change in vaginal depth | Risk of vaginal shortening (e.g., Latzko technique) |
| Use of adjunctive flaps | Omentum, peritoneal flap, rectus abdominus flap | Labial fat pad (Martius fat pad), peritoneal flap, gluteal skin or gracilis myocutaneous flap |
| Relative indications | Large fistulae, location high in a deep narrow vagina, radiation fistulae, failed transvaginal approach, small-capacity bladder requiring augmentation, need for ureteral reimplantation, inability to place patient in the lithotomy position | Uncomplicated fistulae, low fistulae |
The vast majority of VVF in the industrialized world are amenable to a transvaginal repair (Turner-Warwick, 1972; Margolis and Mercer, 1994). The relative advantages of a transvaginal approach compared with an abdominal approach are outlined in Table 77–5 and include shorter operative times, briefer hospital stay, and less blood loss (Goodwin and Scardino, 1979). The principal disadvantages of the transvaginal approach include the relative lack of familiarity of the vaginal cuff anatomy to many urologists; the potential for vaginal shortening, especially with the Latzko approach; and, finally, the difficulty in exposing high or retracted fistulae located near the vaginal cuff, especially in deep, narrow vaginas, or in those without any apical prolapse, such as that found in nulliparous females (see Fig. 77–2). Patients who are unable to get into the high-lithotomy position due to musculoskeletal conditions are not candidates for a transvaginal approach.
Table 77–5 Potential Advantages of a Transvaginal Approach for Posthysterectomy Vesicovaginal Fistula
The abdominal approach to VVF repair is advantageous in several circumstances. If the VVF is associated with other intra-abdominal pathology requiring repair—including an associated ureteral injury (i.e., ureterovaginal fistula), or a complex fistula, including those involving another intra-abdominal organ—then a transabdominal approach is indicated to address the problems simultaneously. If the VVF is located adjacent to the ureteral orifice, some authors have suggested that this is an indication for an abdominal approach (Carr and Webster, 1996), whereas others have not so advocated (Dupont and Raz, 1996). In patients with a small capacity or poorly compliant bladder (often secondary to radiation) requiring augmentation cystoplasty, an abdominal approach is indicated, because both procedures can be performed using the same incision. Complicated fistulae, including those associated with multiple prior failed attempts at repair (Kristensen and Lose, 1994) or those which are quite large (>5 cm), might be best approached abdominally as well. Nevertheless, a prior failed attempt at repair is not necessarily a contraindication to a transvaginal approach because excellent results can be achieved in this setting (Eilber et al, 2003).
Combined transabdominal-transvaginal approaches to VVF have been described (Clark and Holland, 1975; Taylor et al, 1980; Henriksson et al, 1982a). This approach may be applicable selectively in the setting of large, complex, or recurrent VVF after prior attempts at repair.
Handling of Fistula Tract: Excision versus No Excision
A long-held tenet for successful fistula closure, dating back to Sims’ original description in 1852, involves complete excision of the fistulous scar tissue and tract (Fearl and Keizur, 1969; Persky et al, 1979; Wein et al, 1980a; Fourie, 1983). This approach ensures clean, well-vascularized viable edges to be approximated for the initial layer of repair. Simple excision of the scar in an inverted “funnel” shape with careful reapproximation of the defective edges has been shown to be an effective repair of VVF (Iselin et al, 1998; Flynn et al, 2004). However, excision of the fistula tract itself is not always necessary and may even compromise the repair in some patients (Zimmern et al, 1985; Cruikshank, 1988; Tancer, 1992; Raz et al, 1993; Margolis and Mercer, 1994). There are several potential disadvantages of excision of the fistula tract. Firstly, this creates a larger soft tissue defect to be repaired. Excision of the fibrous tract may lead to bleeding, which, if cautery is used, may result in tissue necrosis and impede healing (Eilber et al, 2003). If the VVF is adjacent to the ureter, then excision of the tract may mandate reimplantation of the ureter. Alternatively, if the tract is left in situ, the ureter may be catheterized for the repair and then left undisturbed during a transvaginal operation, obviating the need for reimplantation. In addition, in chronic fistulae, a strong fibrous ring forms outside the epithelialized tract, which maintains some strength through the repair if this layer is incorporated into the closure. This can be an important consideration in those patients with significant detrusor overactivity postoperatively from either the repair itself or the indwelling drainage catheters.
Other Considerations
Preoperative estrogen supplementation may be beneficial in the postmenopausal patient with vaginal atrophy and VVF (Massee et al, 1964). There exists little data in the literature, beyond expert opinion, to support their use. However, topical estrogen preparations may improve vascularity (Margolis and Mercer, 1994) and local tissue quality (Carr and Webster, 1996). Therefore a trial of topical estrogens in individuals with postmenopausal vaginal atrophy and a posthysterectomy VVF may be warranted provided that there are no contraindications to their use.
Perioperative intravenous antibiotics are often administered, although their utility in the posthysterectomy VVF repair is questionable in the absence of infection. Whether antibiotics should be administered prophylactically prior to surgical repair is controversial, but at least one study suggests that preoperative antibiotics do not improve outcomes when administered prior to the repair of obstetric fistula (Tomlinson and Thornton, 1998). Treatment of existing infection based on preoperative urine culture is potentially beneficial in preventing bacteremia during surgery. However, prolonged use of broad-spectrum antibiotics postoperatively following repair may result in bacterial resistance and possibly fungal vaginal infections that may compromise suture lines.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree