(1)
Al Agouza, Cairo, PO, Egypt
6.1 Bladder Agenesis
Incidence
Agenesis of the bladder is an extremely rare congenital anomaly, with approximately 65 cases reported, with only 22 live birth cases to date in the literature written in English [1].
Etiology and Associated Anomalies
The cause of agenesis of the bladder is uncertain, but it may be the result of secondary loss of the anterior division of the cloaca. Bladder agenesis could be induced in experimental animals after Adriamycin exposure [2].
Almost all patients had other associated congenital anomalies especially orthopedic, neurologic, or other urologic anomalies like renal agenesis, dysplastic or ectopic kidneys, ectopic ureters, bicornuate and absent uterus, or vaginal atresia in females and absent prostate, seminal vesicles, or penis in males. Three cases of bladder agenesis in the VATER association have been reported [3].
Unless the ureters are able to drain to an ectopic location such as the vagina, which occurred in the two oldest surviving patients reported, bladder agenesis is incompatible with life. Bladder agenesis is recorded mainly in males, and the reported survived cases are mostly females (Figs. 6.1 and 6.2) [4].
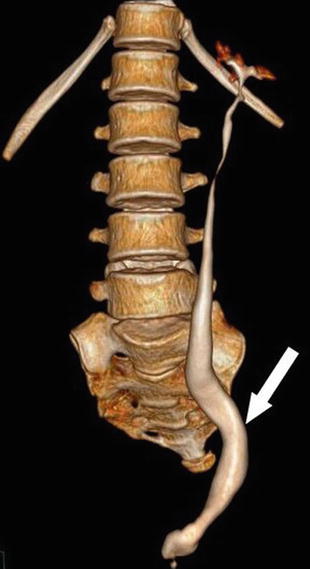
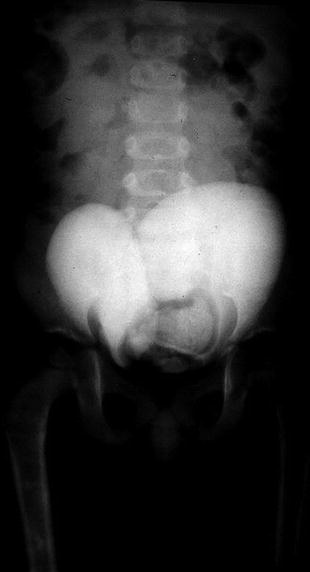

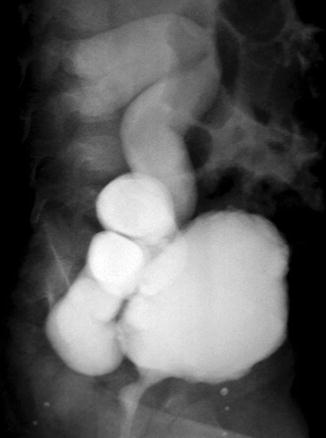
Fig. 6.1
Contrast-enhanced CT abdomen in excretory phase shows lower end of left ureter ending ectopically into the anterior wall of vagina in a case of bladder agenesis [1]

Fig. 6.2
Ascending cystography of bladder duplication
Management
The management of bladder agenesis depends upon the severity and extent of the anomaly; however, the general principle includes relief of obstruction and preservation of renal function by making a continent or non-continent urinary diversion. This can be accomplished by ureterosigmoidostomy, i.e., an internal stoma or an external stoma [5].
6.2 Bladder Duplication
Definition
The presence of two urinary bladder cavities with full-thickness muscular wall, separated by a septum, usually as a part of caudal duplication (dipygus) anomaly [6].
Historical Background
The complete duplication of the bladder and urethra was first reported in a still-born infant in 1871 [7]. Since then, approximately 80 cases have been reported.
Approximate Incidence
This rare anomaly may have an estimated incidence of one in five million. Early reports have shown this anomaly to be more common in males; however, recent reports have shown no specific sex predilection [8].
Associated Anomalies
Bladder duplication is usually associated with other duplication anomalies like hindgut duplication (40–50 %) and duplication of the external genitalia especially in males (90 % double penis and bifid scrotum) [7]. Other associated anomalies include spina bifida, meningocele, or meningomyelocele [8] (Fig. 6.3).
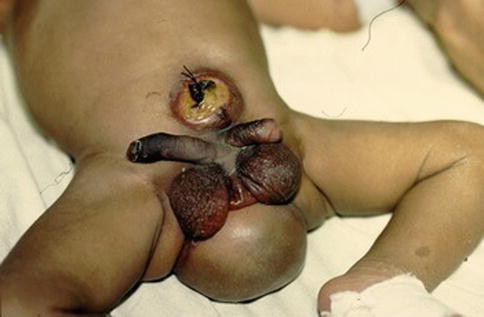

Fig. 6.3
Neonate with complete urinary bladder duplication; note the presence of associated anomalies: omphalocele, double penis, bifid scrotum, and meningomyelocele
Variants of the Condition
Bladder duplication has been described in variants of bladder exstrophy and anomalies of cloacal development [9].
Classification
Abrahamson classified bladder duplication into complete and incomplete duplication. Complete duplication refers to two bladders each emptying through its own urethra which has a separate external urinary meatus. Contrarily, when the two bladders are drained by a single urethra, it is an incomplete type [10].
Complete bladder duplication is further subclassified according to the orientation of the septum intervening between the two bladders into sagittal or coronal, which is the rarest type. The sagittal septum is reported much more common where the two bladders are lying side by side, each receiving the ureter of its ipsilateral kidney and drained by a separate urethra (Fig. 6.4) [10].
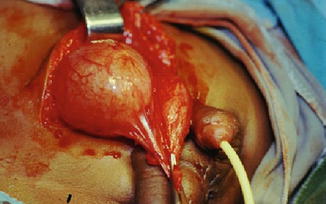

Fig. 6.4
Operative findings in the same patient in Fig. 6.3 showing the two bladders lying side by side divided by a fold of peritoneum and loose areolar tissue
Diagnosis
Clinical features are variable including urinary tract obstruction, incontinence, voiding disorders, and abnormalities of the external genitalia (Fig. 6.3) [11]. Different imaging modalities (ultrasound, cystourethrograms, renal scan, pelviabdominal CT, and spine MRI) are used to evaluate the urinary tract and possible associated anomalies. Urodynamic studies can help to assess the function in both bladder units (Figs. 6.5 and 6.6).
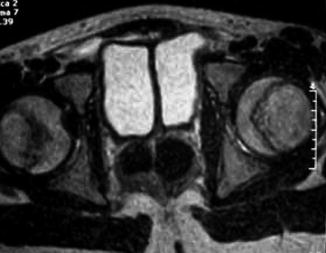
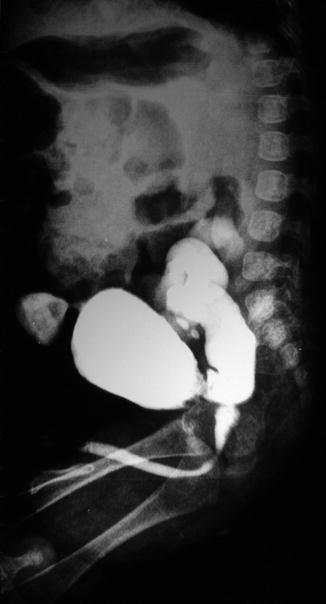

Fig. 6.5
MRI of bladder duplication

Fig. 6.6
Complete bladder duplication with urachal cyst in the anterior moiety
Management
Bladder duplication is quiet consistent with normal life, and treatment is needed if necessary. The goals of treatment are to preserve kidney function and achieve continence and better cosmetic appearance. Delayed treatment is recommended to give time for tailoring an individual treatment plan for each case.
Cystoscopy will help to complete the evaluation of both urethras, bladder necks, and ureteral orifices. Surgical management usually includes excision of the bladder septum while both bladders are united together with ligation of one urethra such that the common bladder will be drained by the more functional urethra.
6.3 Bladder Diverticulum
Definition
Outpouchings of the urothelial mucosa, through the bladder muscular wall.
Historical Background
In 600 BC, the ancient text of Ayurveda on surgery, the Sushruta Samhita, reports bladder stones being removed from bladder “propulsions.” On 1895, Péan first described the surgical management in a 15-year-old female. In 1904, Koller reported the first cystogram demonstrating a bladder diverticulum [12].
Incidence
It occurs in patients of all ages; more than 90 % of patients are males. In a series of over 5,000 children studied, the approximate incidence of congenital bladder diverticulum was 1.7 %. The peak incidence in children is younger than 10 years old [13].
Etiology and Types
Congenital cases are due to:
1.
Failure of muscular bladder wall development: failure of detrusor muscle formation, muscle hypoplasia, or lack of complete formation of a functional muscle layer (primary congenital diverticulum).
2.
Congenital bladder outlet obstruction: in these cases, the diverticula that occur in response to the obstruction serve a beneficial function by acting a pressure pop-off mechanism, protecting the kidney and ureters from high pressures (secondary congenital diverticulum).
Acquired cases are more common and usually due to voiding dysfunctions and acquired bladder outlet obstruction, and this type will not be discussed herein.
Pathology
Distortion of the external surface of the bladder. The excised diverticulum will show a relatively narrow orifice into a large cavity. The diameter is variable ranging from 0.5 to 18 cm, and there may be multiple diverticula. The mucosa adjacent to the diverticulum is hypertrophied or ulcerated [14].
Sites
In pediatric age, it is usually located in the vicinity of the ureteral orifices (Fig. 6.7).
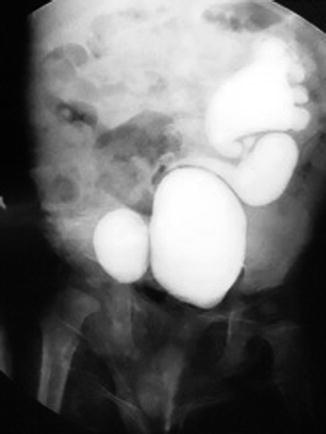

Fig. 6.7
Posterolateral bladder diverticulum
As regard the diverticula in relation to the ureteral orifice, they can be further classified into:
1.
Periureteral: usually associated with normal renal and ureteric development.
2.
Paraureteral diverticula: associated with vesicoureteral reflux (VUR) and therefore may be seen with associated hydronephrosis according to the degree of VUR, whereas periureteral diverticula are associated with both VUR and renal dysplasia, occurring in approximately 15–33 % of patients.
Clinical Picture
Most bladder diverticula are small and asymptomatic and are an incidental finding on bladder imaging or cystoscopy.
When symptomatic in children, the most common presentation is urinary tract infection. Other presentations are hematuria, stones, and urinary retention [16].
Some cases are associated with additional urologic anomalies (urethral strictures and valves, neurogenic bladder, and duplicated collecting system) or syndromes (e.g., Ehlers–Danlos syndrome, prune-belly syndrome, Menkes syndrome, cutis laxa, and Williams syndrome) [17].
Double micturition and residual urine on catheterization after micturition are additional signs, especially with large diverticula.
Ureteral obstruction is unusual, occurring in approximately 5 % of children with bladder diverticulum. However, vesicoureteral reflux is more common, affecting 8 to 13 % of patients (Fig. 6.8).
Several reports have been published describing bladder diverticula presented in inguinal hernia. In infants, the bladder assumes a more abdominal position, which places it in close proximity to the internal inguinal ring. With growth, the pelvis becomes more developed, and the bladder assumes a more pelvic position. Therefore, this is rarely observed in adults. Fact about this entity is important to surgeons during inguinal herniorrhaphy because partial or near total cystectomy may be performed under the mistaken notion that this was a large hernia sac.
Neoplasia occurs in 2–7 % of bladder diverticula; tumors are often large because the location is hidden. All variants of urothelial carcinoma have been reported in bladder diverticula, with a relatively higher frequency of unusual subtypes when compared to the general population [18].
Investigations
Urine analysis and urine C&S to exclude UTI and on US the diverticulum may show echography which reflects the presence of stones or neoplasia.
Voiding cystogram shows the number, location, size, and urinary retention volume of the diverticulum. However, a voiding cystourethrogram with lateral and oblique projections will be indispensable when a urethral obstructive cause is suspected or in congenital cases to rule out possible VUR. CT or MRI scan can also be used in complex malformations or complicated cases.
Cystoscopy will show the diverticular exact site (may be difficult to recognize and concern multiple diverticula), relation to ureteral and urethral orifices, dynamics of the diverticulum during bladder emptying and filling, epithelial changes of the diverticular lining, and the associated bladder changes, e.g., trabeculation and hypertrophy.
Treatment
A vesical diverticulum should never be operated on without previously or simultaneously correcting the cause of obstruction, whether anatomical or functional, that provoked it. A “wait-and-see” approach may be adopted in children with asymptomatic small-sized congenital or paraureteral diverticula and with low-grade associated reflux.
Absolute indications for open surgery are:
1.
The presence of intradiverticular disease (tumor or lithiasis).
2.
Spontaneous diverticular rupture.
3.
Complications related to the size (more than 4 cm diameter) or location of the diverticulum.
Open diverticular excision can be done in three different approaches: extravesical, intravesical, and the combined intravesical and extravesical. Saccules and small diverticula may be treated successfully by electrocoagulation of their mucosa with the ball electrode when the primary obstructive disease is treated. Laparoscopic bladder diverticulectomy is feasible; however, the indications are limited with regard to associated conditions, size, site of the diverticulum, and the child surgical history [19].
Follow-Up and Prognosis
The excision of the diverticulum is generally curative for that particular lesion if it is indicated, although correction of the underlying cause (e.g., outlet obstruction) is required to prevent formation of additional diverticulum (Fig. 6.9).
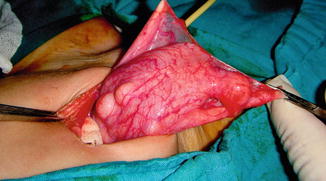

Fig. 6.9
Operative finding of multiple congenital bladder diverticula
6.4 Variant of Bladder Exstrophy
Failure of migration of the mesoderm to the area of the cloacal membrane, and subsequent lack of development of an intermediate layer between its inner (endodermal) and outer (ectodermal) layers, leads to rupture and exposure of an open bladder plate and urethra. These structures occupy a triangular space between a low-set umbilicus superiorly, split rectus abdominis muscles on each side, and an open pelvic ring inferiorly, resulting in a bladder exstrophy.
Nomenclature
Split symphysis variants
Incidence
Bladder exstrophy is a rare anomaly occurring as an isolated defect in 1:30,000–50,000 live births. Variants of the classical bladder exstrophy are very rare (1:400,000–500,000 live births), and they tend to occur more commonly in females, in contrast to the usual male predominance in typical bladder exstrophy; collectively the variant represent 8 % of the cases of bladder exstrophy [20].
Associated Anomalies
Anomalous development of other systems is rare in patients with bladder exstrophy but a variant of exstrophy usually associated with many congenital malformations like bifid scrotum, bilateral cryptorchidism, anorectal malformation, spina bifida occulta, left vertebral hemi-agenesis, and other limb anomalies.
Etiology
The occurrence of exstrophy variants is explained by incomplete rupture or persistence of the abnormal cloacal membrane. The superficial genital organs rarely are involved, and the wedge effect caused by persistence or incomplete rupture of the cloacal membrane may lead to phallic abnormalities.
Classification
Variants of bladder exstrophy are classified as [21]:
Pseudoexstrophy
Covered exstrophy
Duplicate exstrophy
Superior vesical fistula and fissure
Visceral sequestration
OEIS syndrome (omphalocele, exstrophy, imperforate anus, spinal defect)
Bladder Pseudoexstrophy
The term pseudoexstrophy was coined by Hejtamanick et al. in 1954. Pseudoexstrophy is the mildest form of exstrophy variant, and only the musculoskeletal defect is present. The urethra and internal sphincter mechanisms usually are intact, and most of these patients have normal urinary continence [22] (Fig. 6.10).
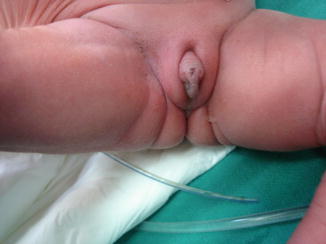

Fig. 6.10
Closed bladder exstrophy in a girl
Covered Exstrophy
Covered exstrophy results from persistence of the infraumbilical cloacal membrane that acts as a wedge, without later rupture. This wedge effect may frequently involve the more superficial perineal structures, leading to phallic abnormalities as well as occasional wide separation of the paired genital (scrotal) swellings.
It may be associated with phallus anomalies as bifid penis or scrotum or sequestrated intestinal remnants at the dome of the bladder. It is less distressing and easier to manage than classical exstrophy of the bladder. Sometimes as in Fig. 6.11 the bladder is seen protruding outside the pelvis with a thin skin coverage (Fig. 6.12).
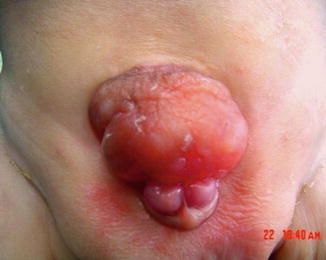
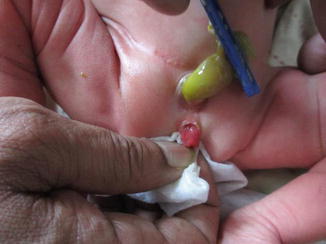

Fig. 6.11
Bladder pseudoexstrophy with epispadias

Fig. 6.12
Closed exstrophy with low insertion of the umbilical cord and epispadias
Genital abnormalities observed in females with covered exstrophy included bifid clitoris (67 %), labial cleft (11 %), hypoplastic labia (11 %), and stenosed duplicate vagina (11 %) [23].
Covered exstrophy differs from the pseudoexstrophy variant in that there is an associated isolated ectopic bowel segment present on the inferior abdominal wall near the genital area. This segment is usually a segment of the colon and has no communication with the underlying gastrointestinal tract. In Fig. 6.13 a baby presented with closed exstrophy with a sequestered colonic tissue between the umbilical contents.
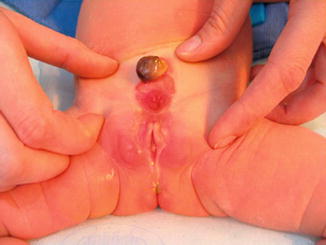

Fig. 6.13
Visceral sequestration between the umbilical vessels with closed bladder exstrophy
Superior Vesical Fissure
In 1974, Williams [24] described a male neonate with epispadias and a segment of colonic remnant attached just below the umbilicus on the abdominal wall.
Superior vesical fissure is a variant in which a pelvic deformity occurs, but only the upper bladder is open near the umbilicus. In a large series of classic exstrophy, only one superior vesical fissure was found among the 72 patients [25] (Fig. 6.14).
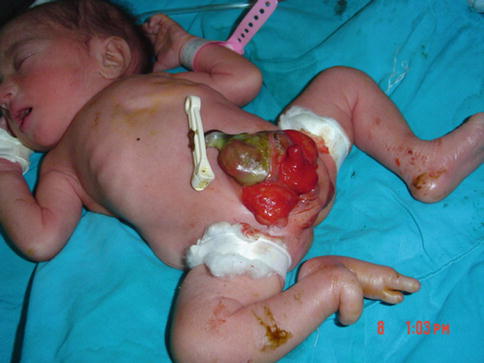

Fig. 6.14
Superior vesical fissure
Because of the presence of a normal urethra in the superior vesical fissure, the long-term prognosis for continence is very high, unlike patients with classic bladder exstrophy. Superior vesical fissure has been described in both male and female subjects, in association with trisomy 18, omphalocele, and duplication of the urethra.
The bladder mucosa on the anterior abdominal wall may increase the patient’s risk of adenocarcinoma of the bladder, more than that seen in classic exstrophy. Most of the cases of adenocarcinoma of the bladder in exstrophy patients occur between the third and sixth decades of life [26].
Duplicate Exstrophy
Duplicate exstrophy is a variant in which the pelvic bones are also abnormal; the bladder is normal but there is a small piece of bladder mucosa on the anterior abdominal wall.
Duplicate exstrophy is one of the rarest variant, and only 23 cases are reported in literature so far [27].
True duplicate exstrophy as defined by Marshall and Muecke refers to a suprapubic exstrophic mucosal plate that is associated with a subjacent covered bladder and a relatively well-formed phallus. The exstrophic plate did not receive ureters and there was no epispadias (Fig. 6.15).
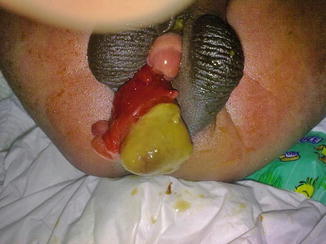

Fig. 6.15
Partially bifid scrotum with intervening partially exstrophic duplicated bladder
Embryologically, it represents part of the spectrum of superior vesical fissure in which the underlying fissure has fused, leaving only an overlying bladder mucosal plate [28].
Pelvic MRI provides adequate information about the internal genitalia before and after surgery; therefore, MRI is mandatory before repair.
Omphalocele Exstrophy, Imperforate Anus, and Spinal Defect (OEIS Syndrome)
The OEIS complex comprises a combination of defects including omphalocele, exstrophy of the cloaca, imperforate anus, and spinal defects. It may represent the most severe manifestation of a spectrum of birth defects and the exstrophy–epispadias sequence.
Opinions differ about the possible existence of a continuum of abnormalities ranging from epispadias to bladder exstrophy to cloacal exstrophy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree