Fig. 26.1
A retrograde ureteropyelogram demonstrates a 3 cm ureteral tumor
After examining the lower urinary tract, the entire upper urinary tract is thoroughly surveyed using one of two “no touch techniques.” This refers to visual inspection of the entire urothelium prior to any instrumentation; even guidewire introduction may incur trauma and cause bleeding, which impairs visualization and confounds inspection. Using the first “no touch technique,” a small caliber semirigid ureteroscope is inserted into the bladder and used to cannulate the ureteral orifice under direct vision [62, 63]. The scope is advanced as far proximally as possible without significant torque or angulation. While in a female the semirigid ureteroscope may often be passed to the renal pelvis without difficulty, the scope typically cannot be passed beyond the level of the mid-ureter at the iliac vessels in most patients. The initial use of a radially dilating semirigid ureteroscope to inspect the distal and mid ureter facilitates subsequent insertion of the flexible ureteroscope (FU). The semirigid ureteroscope is then removed and a guidewire is left in place at the level last inspected. Over this wire, a FU is inserted under fluoroscopic guidance. At this step, care must be taken to prevent inadvertent proximal migration of the wire, which again may cause urothelial injury. Dilation of the ureter should also be avoided to minimize the risk of trauma from both the guidewire and the dilation itself. Due to the miniaturization of ureteroscopes, it is rarely necessary to formally dilate the ureter. In fact, Hudson et al. found that the ideal FU outer diameter is 7.5 F to minimize the need for ureteric dilation, as <1% of cases failed to pass without formal dilation using this size scope [64]. However, digital ureteroscopes have a distal digital chip-based camera (CCD or CMOS), or “chip on the tip” technology which necessitates a larger endoscope outer diameter of nearly 10 F and therefore may require dilation before scope insertion.
Utilizing the second “no touch technique,” the semirigid ureteroscope is not used at all. Instead, the FU is exclusively employed for the entire inspection using a wireless, sheathless approach [65]. Under direct vision, the FU is inserted into the bladder, throughout the ureter, and the intra-renal collecting system. Importantly, this is only possible with the appropriately sized endoscopes and a patent ureteral lumen.
The FU is then advanced under direct vision throughout the ureter and any small ureteral tumors are biopsied and treated as they are encountered using the techniques described below (Fig. 26.2). Commonly, such small lesions may be avulsed with scope advancement, which may induce bleeding and/or make it difficult to identify the lesions later during the procedure. If a large ureteral tumor is encountered, it may be helpful to advance the ureteroscope beyond the lesion to survey the more proximal ureteral segment to identify other tumors. In some instances, it may be difficult to identify the ureteral lumen itself to pass the tumor. It can be useful to follow the contour of the ureteral wall and, in select cases, to pass a hydromer-coated guidewire beyond the tumor to gain proximal access. While a no touch technique is preferred, a guidewire is utilized anytime there is concern in being able to reaccess the ureter.
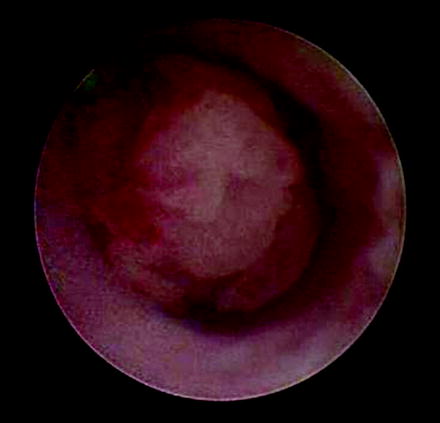

Fig. 26.2
Endoscopic view of ureteral neoplasm
Next, the collecting system is entered and a renal pelvis aspirate is obtained and sent fresh in normal saline for cytology. This is done by connecting a large Luer lock syringe (50–60 mL) directly to the irrigation port (Fig. 26.3). Then, systematic pyeloscopy is performed and the entire intra-renal collecting system is inspected. Severe deflection may be required to reach lower pole calices and thereby induce premature bleeding. For this reason, inspection should proceed in a cranial to caudal fashion in order to minimize bleeding and optimize visualization. In order to inspect the lower pole calices in most kidneys, the ureteroscope must deflect at least 170° [61]. However, in most currently available fiberoptic ureteroscopes, the deflection has been increased to approximately 300° to compensate for the loss of deflection with working instruments in place.
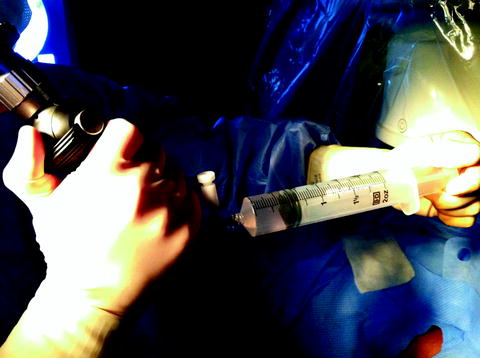

Fig. 26.3
Obtaining a renal pelvis aspirate by connecting a large Luer lock syringe (50–60 mL) directly to the irrigation port of the flexible ureteroscope
Following a survey of the entire intra-renal collecting system, any tumors are then sampled for microscopic analysis. Over the years, various devices have been introduced to biopsy upper tract tumors including baskets, forceps, brushes, snares, and graspers. The two most commonly utilized devices include the 3 F cup biopsy forceps and the stainless steel flat wire basket (Fig. 26.4) [62]. The small cup forceps are available in several designs of 1-mm diameter. These are ideally suited for sampling smaller, sessile, or nonpapillary lesions. If the sample is entirely contained within the cup, the device may be withdrawn through the working channel. Then, the device is replaced and multiple samples are taken in a similar fashion to ensure sufficient specimen for cytologic evaluation. However, if the tissue fragment extends beyond the extent of the cup, the entire unit consisting of FU, forceps, and biopsy should be removed to preserve the largest specimen possible. The endoscope is then replaced and additional biopsies are obtained. Depending on the FU utilized, the shaft stiffness of the cup forceps often limits the deflection to approximately 90–100° [61]. This can pose obvious challenges when attempting to biopsy lower pole lesions.


Fig. 26.4
The two most commonly used ureteroscopic biopsy devices are the cup biopsy forceps and flat wire basket
Alternatively, the stainless steel flat wire basket is effective for more sizable, papillary, and friable tumors [62]. The tumor is trapped between the angles of the wire. Next, the basket is closed snugly but not completely. It is then used to avulse a piece of tissue. In this way, a large sample measuring up to several millimeters may be retrieved. In contrast to the cup forceps, samples obtained with the flat wire basket should not be withdrawn through the working channel since most of the tissue would be sheared off and lost. Instead, the entire unit consisting of FU, basket, and biopsy should always be withdrawn to maintain the largest tissue specimen possible (Fig. 26.5). Again, the FU is reinserted and multiple biopsies are taken. Using the flat wire basket, large tumors can be mechanically debulked quite effectively. In fact, the flat wire basket may be utilized to biopsy tumors measuring several centimeters in diameter.
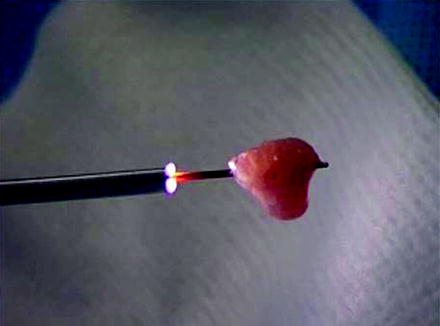

Fig. 26.5
A large piece of tumor is shown hanging from the flat wire basket. The entire unit consisting of tumor, basket, and ureteroscope is withdrawn to preserve the largest specimen possible
Other devices are largely ineffective and thus seldom used. Unlike the flat wire basket, the nitinol round wire baskets do not have enough grasping edge to capture tumor well. The brush is also very poor in retrieving tissue. When viewed directly endoscopically, the brush can be seen moving the tissue but not actually sampling it. Wire pronged graspers may be useful for papillary tumors if other devices are not available or cannot reach the tumor. Recently, a larger cup forceps with a 2 mm cup has been introduced (Fig. 26.6). However, two key points warrant discussion. First, it must be back-loaded through the working channel of the FU. In turn, the back-loading biopsy forceps and the FU must be placed through a ureteral access sheath. If this device is employed, we recommend first carefully inspecting the ureter with a no touch technique to evaluate for ureteral lesions prior to access sheath insertion. Second, the larger cup forceps obscure the visual field when opened (Fig. 26.7). Despite these obvious disadvantages, the larger cup forceps appear to offer better deflection characteristics compared to the small cup forceps and flat wire basket (Fig. 26.8). However, the amount of tissue retrieved using the flat wire basket is significantly larger (Fig. 26.9).

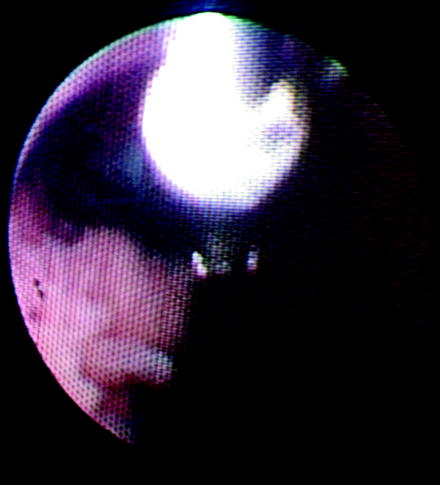
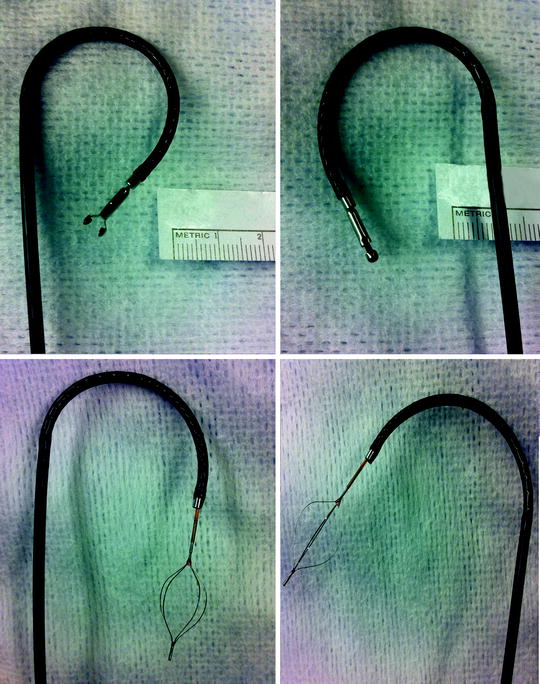
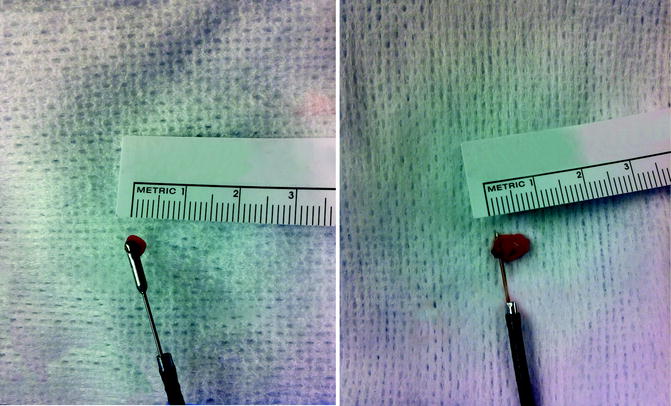

Fig. 26.6
Back-loading biopsy forceps with 2 mm cup

Fig. 26.7
The visual field is obscured by the back-loading biopsy forceps

Fig. 26.8
Superior deflection characteristics of the back-loading biopsy forceps compared to the flat wire basket

Fig. 26.9
Larger sample obtained by using flat wire basket compared to back-loading biopsy forceps
After ureteroscopic biopsy, the tissue sample is transferred directly from the cup forceps or flat wire basket into a specimen container with normal saline (Fig. 26.10). By minimizing the number of specimen transfers, the chance of specimen loss is reduced. Cytologic preservative known as Saccomanno fixative is added if specimen delivery to the Cytopathology laboratory may be delayed. Fresh specimens are hand delivered to the laboratory, where they are evaluated using the cytospin smear technique. Importantly, all samples are processed as cytology specimens to avoid tissue loss during preparation [66]. Cytopathologic techniques for handling and interpreting the ureteroscopic biopsy specimens will be discussed in detail later in this chapter. Multiple samples are taken with either device to obtain sufficient tissue for pathologic analysis. This includes not only multiple samples of the tumor itself, but also post-biopsy and post-laser aspirates.
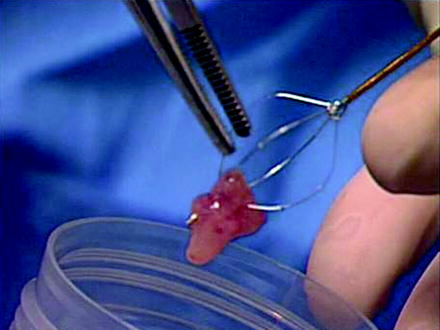

Fig. 26.10
The specimen is transferred directly into a small specimen cup containing normal saline without fixative solution and then delivered to the Cytopathology laboratory
Once adequate tissue sample has been obtained, the tumor is then treated. Advances in laser technologies have transformed the endoscopic management of upper tract tumors. In particular, the holmium (Ho) and neodymium (Nd):yttrium aluminum garnet (YAG) lasers allow for the safe and efficacious treatment of UTUC [61]. Laser ablation and resection will be described in detail in subsequent chapters. Briefly, the Ho:YAG laser is a pulsed device with a wavelength of 2,100 nm. Because the holmium energy penetrates tissue only 0.5 mm, its tissue effect is relatively superficial and visible endoscopically. The Ho:YAG laser has a dominant ablative effect, but may also be used to coagulate tumors by defocusing the laser beam on the tissue. On the other hand, the Nd:YAG laser is a continuous wave device with a wavelength of 1,064 nm. This penetrates ten times deeper than Ho:YAG, with a depth of penetration up to approximately 5–10 mm. Unlike Ho:YAG, it is more difficult to determine the depth of penetration during treatment. Due to the increased risk of ureteral stricture formation, use of Nd:YAG should be avoided within the ureter. Even within the kidney, Nd:YAG should be applied cautiously as indiscriminate use may lead to infundibular stenosis. While the Ho:YAG both ablates and coagulates tissue, the Nd:YAG only coagulates. However, it is particularly effective at coagulating bleeding sites and large tumors up to several centimeters in diameter. In contrast to the Ho:YAG which is a contact laser, the Nd:YAG does not require direct contact of the laser fiber with the neoplasm. This property may be advantageous for tumors which are difficult to reach. These two lasers are best used in combination. In fact, the Ho and Nd:YAG lasers are available in a combined unit with a dual foot switch. This allows for rapid interchange and selective application of each laser to exploit their unique properties. Most commonly, a 200 or 365 μm laser fiber is employed.
Notably, special Ho:YAG lasers have been developed with variable pulse duration [61]. While the typical pulse duration for holmium laser lithotripsy of urinary calculi is 350 μs, the variable pulse duration laser allows for the pulse length to be increased to 700 μs. The longer pulse delivers the same energy over a longer time, which in turn improves coagulation. Hence, this type of holmium laser is ideal for treating ureteral tumors.
Specimen Handling
By the end of the procedure the following samples should be obtained:
1.
Bladder urine.
2.
Saline wash and aspirate from site of lesion(s).
3.
Biopsy of the lesion(s) in saline.
4.
Post-biopsy aspirate.
5.
Post-laser aspirate.
One of the most crucial principles in handling the tissue for pathologic diagnosis is close collaboration with the cytopathologist. Delivering the specimens without fixation, fresh in saline, to the Cytopathology laboratory is of utmost importance for tissue preservation. Samples are prepared with the cytospin technique. If there is any macroscopically visible tissue in the sample, a cell block is also prepared. This sample often demonstrates both the architecture of the neoplasm and the individual cells, to allow grading of urothelial carcinoma.
Cytospin Technique
First, Saccomanno fixative is added to the specimen. If the specimen appears grossly hematuric, a Cytorich red solution is first added in order to lyse the red blood cells. The specimen is centrifuged for 10 min at 1,200 RPM. Most of the supernatant is then discarded. Approximately 3–5 mL of the supernatant is placed into a special funnel apparatus, which contains a specimen slide, and centrifuged for 10 min at 1,300 RPM. At the end of this process, the cells are adherent to the slide. The slide is disassembled and air dried for 10 min, placed in alcohol 95% to remove the carbowax contained in the Saccomanno fixative and is then inserted into the machine for Papanicolaou staining. Finally, the slide is placed in Xylene bath for 2 min for clearing of alcohol and coverslipped (Fig. 26.11a).
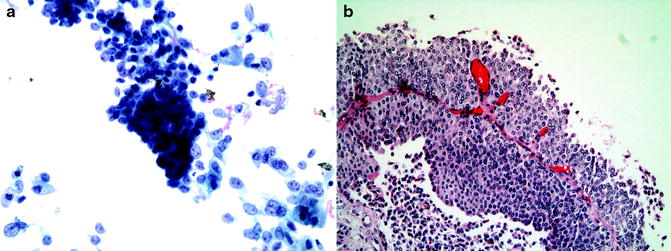

Fig. 26.11
(a) Grade 2 tumor in cytology specimen. (b) The cell block shows the histology of the same tumor
Cell Block Preparation
A cell block can be prepared whenever there is any visible tissue in the sample. This is done using the pellet obtained following the first centrifugation. The pellet is transferred to a fenestrated bag and wet with formalin. The bag is wrapped and placed in a cassette, which is immersed in Formalin solution. The specimen is processed in several solutions, including two fixations in Formalin, dehydration from 70% alcohol through three absolute alcohol solutions, and clearing of alcohol in Xylene solution. It is then extracted from the bag and embedded in wax, sliced, and adhered to a glass slide. Lastly, the slides are stained with hematoxylin and eosin (H&E) and prepared as a histologic specimen. Architectural details of the lesion can often be demonstrated using cell block preparation (Fig. 26.11b).
Using this systematic approach to ureteroscopic biopsy and specimen preparation, we have significantly improved our diagnostic yield. By handling all specimens in the Cytopathology laboratory, we increased our yield for diagnostic yield from 40% to approximately 90% [66].
There has been concern that the ureteroscopic biopsies may not reflect the actual pathology, particularly the tumor grade. However, considering tumor grade, several series have demonstrated a biopsy concordance of approximately 80–90% between the endoscopic and final pathologic specimens [34, 35]. Moreover, the accuracy may be enhanced by providing the pathologist with multiple samples and, ideally, macroscopically visible samples to be used for cell block preparation. Importantly, all samples should be delivered to the Cytopathology laboratory in normal saline as rapidly as possible to keep them fresh without cellular degradation (Tables 26.1 and 26.2).
Table 26.1
Samples collected for cytology during ureteroscopic biopsy
1. Bladder urine |
2. Aspirate of ureter or renal pelvis urine |
3. Biopsy of tumor(s) |
4. Post-biopsy renal pelvis aspirate |
5. Post-laser renal pelvis aspirate |
Table 26.2




Handling of biopsy specimens
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







