Chapter 49 Unexplained Acute Pancreatitis
Determining the cause of acute pancreatitis is not usually difficult. Acute pancreatitis results most commonly from alcohol abuse or gallstone disease. These etiologies account for 60% to 90% of the cases (Box 49.1).
Alcoholism is diagnosed by history, and gallstones are diagnosed by a combination of demographic characteristics, laboratory findings, and radiographic imaging studies. Other causes of pancreatitis include hypertriglyceridemia, hypercalcemia, drug reactions, trauma, surgery, and endoscopic retrograde cholangiopancreatography (ERCP). In these cases the relationship of the episode of pancreatitis to the cause is usually clear. However, despite a careful history, physical examination, laboratory testing, and radiologic evaluation, a cause for acute pancreatitis will not be identified in 10% to 30% of patients.1 These patients are conventionally classified as having idiopathic acute pancreatitis (IAP).2,3 When patients have more than one clinical episode of idiopathic pancreatitis they are given the diagnosis of idiopathic acute recurrent pancreatitis (IARP).2–7 Evaluation and therapy is important because more than 50% of untreated patients with IARP experience recurrent episodes that may lead to chronic pancreatitis.5,8 This chapter reviews the role of ERCP with ancillary endoscopic techniques, endoscopic ultrasound (EUS), and magnetic resonance cholangiopancreatography (MRCP) in the evaluation and therapy of patients with IAP and IARP. The reader should be aware that significant controversy exists as to the appropriate use and timing of ERCP in IAP because the available evidence is often sparse and largely uncontrolled.
Pathophysiology and the Role of ERCP, EUS, and MRCP
The literature suggests that the pathophysiologic process of acute pancreatitis might consist of three phases. The initial phase involves triggering events that are, for the most part, extrapancreatic in origin. Clinically, the most important of these appears to be either passage of a biliary tract stone or ingestion of ethanol. Other events, such as exposure to pancreatotoxins, pancreatic ischemia, and infection, may also be capable of triggering acute pancreatitis. The second phase involves a series of events that occur within the acinar cells of the pancreas. Finally, a third phase consisting of both acinar cell and nonacinar cell events occurs and determines the ultimate severity of an attack of pancreatitis. The two key points in these events that are important with regard to endoscopic intervention are (1) pancreatic ductal obstruction leads to ductal hypertension that is exacerbated by pancreatic secretion and (2) ductal hypertension causes inhibition of enzyme secretion resulting in colocalization of inactive pancreatic enzymes and lysosomal hydrolases with subsequent acinar cell injury and the clinical sequelae of acute pancreatitis.9 Thus the role of ERCP, EUS, and MRCP in patients with IAP and IARP is to identify causes of triggering events that resulted in the pancreatic ductal obstruction with the therapeutic goal of relieving the obstruction. It is assumed that relief of the obstruction will prevent further episodes of pancreatitis. The obstructive theory of IAP also assumes that ductal obstruction is intermittent or that a second risk factor predisposes patients with impaired ductal drainage.10
Diagnostic Findings and the Timing of ERCP, EUS, and MRCP
There are two major concerns that prompt physicians to do a more intensive evaluation of the patient with acute pancreatitis in whom no obvious cause is found. The first is that the patient may have an underlying disease that will predispose to further attacks of acute pancreatitis unless the cause is identified and adequately treated. Acute pancreatitis is likely to recur in 33% to 67% of patients with biliary tract disease when not diagnosed and treated.11 Similarly, other anatomic or functional disorders of the pancreaticobiliary tree may predispose patients to recurrent episodes of pancreatitis. The second concern is that the pancreatitis may be related to a tumor. As a result, ERCP, EUS, and MRCP now play a central role in the evaluation and therapy of patients with IAP.
In the past when EUS and MRCP were not widely available, ERCP was considered a reasonable option to investigate IAP and IARP, as it both was diagnostic and offered therapeutic options. There are a number of potential causes of IAP that can be diagnosed and potentially treated by ERCP and ancillary techniques. These include occult gallstones, abnormalities and anomalies of the pancreatic duct and bile duct, sphincter of Oddi dysfunction, and ampullary and pancreatic neoplasms. The techniques applied at ERCP to diagnose the cause of IAP are shown in Box 49.2.
Box 49.2
Techniques Applied at ERCP to Diagnose Cause of Idiopathic Acute Pancreatitis
Although there are potential gains by performing ERCP (identifying and treating the cause and preventing another episode of acute pancreatitis), there are potential downsides for the patient and the health care system as a whole (the inappropriate performance of the procedure and its adverse events).12 The timing of ERCP in patients with IAP is controversial. Ballinger and colleagues reported that only 1 of 27 patients with one unexplained episode of pancreatitis and the gallbladder in situ had a second episode of pancreatitis during a 3-year follow-up period.2 They felt that the risks of ERCP were greater than the risks of a second episode of acute pancreatitis and advised against its use. On the other hand, Trapnell and Duncan reported that 35 of 148 patients (24%) with IAP suffered a recurrence, but if gallstones were present, the rate increased to 38%.13 Using an actuarial method, the authors found that 10% of patients with IAP were likely to have a first recurrence within 1 year of the initial attack, 17% within 2 years, and 25% within 6 years. Patients who had one recurrence were likely to have a second. In a cost-utility analysis, Gregor and colleagues found that performing ERCP on all patients after a first episode of idiopathic pancreatitis was neither of great benefit nor particularly cost effective.12 However, it is of substantial benefit and cost effective in a subgroup of patients with greater probability of having an occult stone.
EUS and MRCP have assumed a central role in the evaluation of patients with IAP and IARP because of their high diagnostic accuracy and low morbidity.14 The use of secretin in both EUS (S-EUS) and MRCP (S-MRCP) has further improved the diagnostic yield for identifying underlying structural etiologies for IAP.15 The results of EUS and MRCP can direct an alternative therapy (e.g., cholecystectomy for microlithiasis) and obviate the need for a more invasive ERCP. The limitations of these two diagnostic modalities are that they have no therapeutic options and a separate procedure may have to be carried out for treatment. ERCP is now used primarily as a therapeutic option directed by the results of EUS and MRCP unless the EUS and MRCP are negative. In such a setting, sphincter of Oddi manometry is usually combined with the ERCP.
Our approach is (usually) to evaluate patients presenting with IAP and IARP with EUS and/or S-MRCP. For patients aged 40 years and older in whom findings are negative or inconclusive, we would then proceed to performing ERCP (usually with sphincter of Oddi manometry and bile microscopy when the gallbladder is in situ) after their first episode of acute pancreatitis. This is based on the findings of Choudari and colleagues.16 In their study, 21% of patients aged 40 to 60 years and 25% of patients older than 60 years had a neoplastic process as the cause of their pancreatitis, in contrast to only 3% younger than 40 years (Table 49.1).
Table 49.1 Idiopathic Acute Pancreatitis: Yield of ERCP with or without Sphincter of Oddi Manometry and with or without Bile Microscopy Correlated with Age (n = 225)
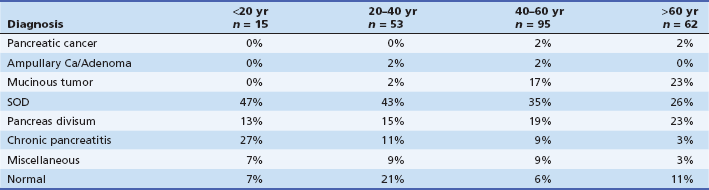
From Choudari CP, Fogel EL, Sherman S, et al. Idiopathic pancreatitis: yield of ERCP correlated with patient age. Am J Gastroenterol. 1998;93:1654A.
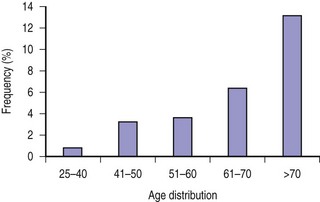
In another similar series of 1241 idiopathic pancreatitis patients reported by Fischer and colleagues, the incidence of malignancy and premalignant conditions was 4.7% (58 of 1241).17 Analysis of intraductal papillary mucinous neoplasm (IPMN), which accounted for 52 of 58 of the malignant and premalignant conditions, showed an increasing trend with age ranging from 1.3% in patients younger than 40 years to 13% in patients older than 70 years (Fig. 49.1).
Occult Gallstone Disease
Although microlithiasis and biliary sludge are technically different, the terms are often used interchangeably. Microlithiasis most commonly refers to stones <3 mm in diameter and biliary sludge is considered to be a suspension of crystals, mucin, glycoproteins, cellular debris, and proteinaceous material within bile.18 The crystals are made of cholesterol monohydrate, calcium bilirubinate, or calcium carbonate. Microlithiasis and biliary sludge can be found within the gallbladder or bile ducts and they may not be detected by standard gallbladder imaging techniques such as ultrasound or computed tomography (CT) scan.
Microlithiasis of the gallbladder has been implicated as a common cause of IAP. Two prospective studies found that approximately two thirds to three fourths of patients with IAP harbored occult gallstones in the gallbladder.7,19 The diagnosis was based on microscopic examination of bile for crystals and usually confirmed on evaluation of the resected gallbladder or follow-up gallbladder ultrasound showing gallstones and/or sludge. On multivariate analysis, the finding of biliary crystals in bile is a strong predictor of small stones or sludge in the gallbladder (p <0.001).19 Moreover, the finding of crystals in bile had a sensitivity of 86%, a specificity of 86%, and a positive predictive value of 92% for the diagnosis of gallstone disease as the missed cause of IAP. In contrast to the results of Lee and colleagues and Ros and colleagues, some investigators have detected microlithiasis in fewer than 10% of patients with IAP.5,7,19,20 Bile may be collected at the time of ERCP from the duodenum or bile duct after gallbladder stimulation with cholecystokinin or by direct cannulation of the gallbladder.
In patients with IAP, EUS may help identify patients with underlying microlithiasis when conventional transcutaneous ultrasound is normal.21–23 Frossard and colleagues reported that EUS identified a biliary cause of IAP in 103 of 168 patients (61%). Of these 103 patients, 52 (50%) had gallstones or microlithiasis, 12 (12%) had gallbladder sludge, 10 (10%) had common bile duct stones and 29 (28%) had a combination of these findings.24 Yusoff and colleagues compared the diagnostic yield of EUS in 201 patients with a single episode of IAP with 169 patients with recurrent episodes. Biliary tract disease (46 of 246; 18.7%) was the most common positive finding in the patients with gallbladder in situ. In postcholecystectomy patients, 4 of 124 (3.2%) had evidence of stones in the bile duct.25 Morris-Stiff and colleagues showed that in 42 patients with idiopathic pancreatitis and a normal MRCP, EUS detected cholelithiasis or microlithiasis alone in 9 patients (21.4%), cholelithiasis and choledocholithiasis in 6 patients (14.3%), and choledocholithiasis alone in 1 patient (2.4%).26 In another series of 44 patients with IAP, EUS found cholelithiasis in 3 patients (6.8%), microlithiasis in 20 patients (45.5%), and choledocholithiasis in 2 patients (4.5%).27 Ardengh and colleagues found gallbladder microlithiasis in 27 of 36 patients with IAP (75%). When compared to the final surgical resection specimen, the sensitivity, specificity, and positive and negative predictive values to identify gallbladder microlithiasis were 92.6% (range of 74.2% to 98.7%), 55.6% (range of 22.7% to 84.7%), 86.2% (range of 67.4% to 95.5%), and 71.4% (range of 30.3% to 94.9%), respectively. Overall EUS accuracy was 83.2%.28
Intraductal ultrasound (IDUS) has also been shown to be useful in picking up occult stones, microlithiasis, and biliary sludge within the bile ducts.29–32 The study by Kim and colleagues32 specifically looked at 31 patients with idiopathic recurrent pancreatitis with negative findings on ERCP. IDUS revealed small bile duct stones (≤3 mm) in 5 patients (16.1%) and sludge in 3 patients (9.7%).
The role of MRCP in detecting choledocholithiasis is well established.31,33 Its role in detecting microlithiasis or sludge of the gallbladder has not undergone extensive study. Calvo and colleagues showed that in 80 patients undergoing both transcutaneous ultrasound and MRCP for suspected gallstones, the sensitivity of MRCP in diagnosing gallbladder stones (43 patients; 97.7%) was comparable to transabdominal ultrasound (44 patients). MRCP diagnosed biliary sludge or microlithiasis in 13 patients, versus 5 in the case of ultrasound. The authors concluded that MRCP is a good technique for diagnosing cholelithiasis and biliary sludge. However, its high cost, contraindications, and the need for patient cooperation limit the use of the technique for routine clinical gallbladder evaluation.34
Treatment of microlithiasis can significantly reduce the incidence of recurrent pancreatitis.7,19 There are several therapeutic options for managing patients with pancreatitis due to microlithiasis. Laparoscopic cholecystectomy should be considered the procedure of choice, as it is almost always curative. Ros and colleagues reported no further episodes of pancreatitis in 17 of 18 patients followed for 3 years after cholecystectomy.19 Endoscopic biliary sphincterotomy is an excellent alternative for the elderly and high-risk surgical patient.11 Dissolution therapy with ursodeoxycholic acid has also been shown to prevent recurrent pancreatitis.11 However, maintenance therapy appears necessary to prevent recurrent stone formation.
Given the high prevalence of occult microlithiasis in some series, some authors advocate empirical cholecystectomy as a first-line therapy, particularly in patients with recurrent attacks.35,36
Sphincter of Oddi Dysfunction
Sphincter of Oddi dysfunction (SOD) refers to the abnormality of SO contractility that is manifested clinically by pancreaticobiliary pain, pancreatitis, or deranged liver function tests. Sphincter of Oddi manometry (SOM) is considered by most authorities to be the gold standard test for diagnosing SOD.37–41 SOM can be performed percutaneously or intraoperatively but is most commonly performed at the time of ERCP. SOM uses a water-perfused catheter that is inserted into the common bile duct, pancreatic duct, or both to measure sphincter pressure. The diagnosis of SOD is established when the basal pressure is equal to or greater than 40 mm Hg.39
Because SOM is difficult to perform, invasive, not widely available, and associated with a high adverse event rate, several noninvasive and provocative tests have been designed in an attempt to identify patients with SOD. The data currently available suggest that these tests lack the sensitivity and specificity to replace SOM.38,42
The role of S-MRCP in diagnosing SOD is still debatable. Mariani and colleagues reported that S-MRCP and SOM were concordant in 13 of 15 patients (86.7%).43 However, subsequent larger studies did not show this high concordance. Pereira and colleagues showed a diagnostic accuracy of S-MRCP for SOD type II and III of 73% and 46%, respectively.44 Aisen and colleagues also showed that there was no difference in the magnitude of the increase of pancreatic duct diameter between patients with elevated basal sphincter pressure and normal basal sphincter pressure.45
The role of S-EUS in diagnosing SOD in idiopathic pancreatitis is even more unclear. The study by Mariani and colleagues labeled two patients with SOD based on the fact that there was persistent main pancreatic duct dilation 15 minutes after secretin injection. They confirmed the diagnosis by showing that there was no recurrence of pancreatitis 18 months after sphincterotomy was performed.15 To date there have been no studies comparing S-EUS and SOM, which is the gold standard for diagnosing SOD.37–41
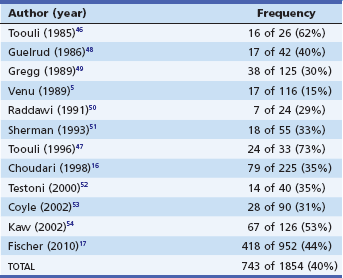
SOD is a frequent cause of idiopathic acute pancreatitis. It has been manometrically documented in 15% to 73% of such patients (Table 49.2).5,16,17,46–54
Table 49.2 Manometrically Documented Sphincter of Oddi Dysfunction Causing Idiopathic Acute Pancreatitis
Pancreatic sphincter manometry should be done in patients with IARP, particularly those with normal biliary manometry and those who have recurrent attacks after a biliary sphincterotomy. It is not surprising that isolated pancreatic sphincter hypertension is common among patients with IARP found to have SOD.10,50 Also, pancreatic sphincter dysfunction may explain recurrent pancreatitis despite biliary sphincterotomy or surgical biliary sphincteroplasty.10
Sphincter ablation is the recommended therapy for patients with recurrent pancreatitis due to SOD. Historically, this has been done surgically.55,56 However, with the increasing experience, endoscopic sphincterotomy has become the treatment of choice.
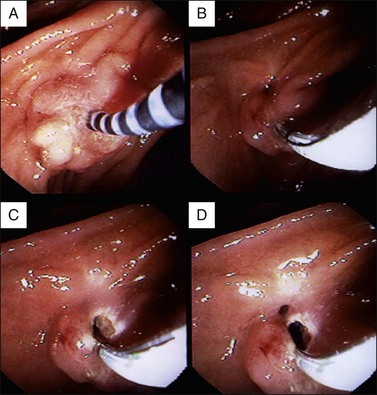
The value of ERCP, SOM, and sphincter ablation therapy was studied in 51 patients with idiopathic pancreatitis.57 Twenty-four patients (47.1%) had an elevated basal sphincter pressure. Thirty were treated by biliary sphincterotomy (n = 20) or surgical sphincteroplasty with septoplasty (n = 10). Fifteen of 18 patients (83%) with an elevated basal sphincter pressure had long-term benefit (mean follow-up of 38 months) from sphincter ablation therapy (including 10 of 11 treated by biliary sphincterotomy) in contrast to only 4 of 12 (33.3%, p <0.05) with a normal basal sphincter pressure (including 4 of 9 treated by biliary sphincterotomy). However, Guelrud and colleagues found that severance of the pancreatic sphincter was necessary to resolve the pancreatitis (Fig. 49.2).58
In this series, 69 patients with idiopathic pancreatitis due to SOD underwent treatment by standard biliary sphincterotomy (n = 18), biliary sphincterotomy with pancreatic sphincter balloon dilation (n = 24), biliary sphincterotomy followed by pancreatic sphincterotomy in separate sessions (n = 13), or combined pancreatic and biliary sphincterotomy in the same session (n = 14). Eighty-one percent of patients undergoing pancreatic and biliary sphincterotomy had resolution of their pancreatitis compared to 28% of patients undergoing biliary sphincterotomy alone (p <0.005). Sherman and colleagues reported that only 44% of SOD patients with IARP had no further attacks during a 5-year follow-up interval after biliary sphincterotomy alone.51 These data are consistent with the theory that many such patients who benefit from biliary sphincterotomy alone may have subtle gallstone pancreatitis or that perhaps the follow-up has not been long enough to detect another attack of pancreatitis. A recent paper by Wehrmann attempted to clarify the issue by studying patients with a longer follow-up (11.5 +/− 1.6 years) after endoscopic therapy in SOD for IARP. In this study, 5 of 37 patients (14%) had a recurrent attack of pancreatitis over a mean duration of 32.4 months (range of 24 to 53 months) and this increased to 19 of 37 (51%) at 11.5 years. However, the frequency of pancreatitis episodes were lower than prior to endoscopic therapy. The authors suggest that endoscopic sphincter ablation may slow the progress of the natural course of the disease.59
The results of Guelrud and colleagues’ study also support the anatomic findings of separate biliary and pancreatic sphincters and the manometry findings of residual pancreatic sphincter hypertension in more than 50% of persistently symptomatic patients who undergo biliary sphincterotomy alone.58 Kaw and colleagues reported that among patients with idiopathic pancreatitis secondary to SOD, 78% had persistent manometric evidence of pancreatic sphincter hypertension despite a biliary sphincterotomy.54 Toouli and colleagues demonstrated the importance of pancreatic and biliary sphincter ablation in patients with idiopathic pancreatitis.47 In this series, 23 of 26 patients (88%) undergoing surgical ablation of both the biliary and the pancreatic sphincter were either asymptomatic or had minimal symptoms at a median follow-up of 24 months (range of 9 to 105 months). Okolo and colleagues retrospectively evaluated the long-term results of endoscopic pancreatic sphincterotomy in 55 patients with manometrically documented or presumed pancreatic sphincter hypertension (presumption based on recurrent pancreatitis with pancreatic duct dilation and contrast medium drainage time from the pancreatic duct greater than 10 minutes).60 During a median follow-up of 16 months (range of 3 to 52 months), 34 patients (62%) reported significant pain improvement. Patients with normal pancreatograms were more likely to respond to therapy than those with pancreatographic evidence of chronic pancreatitis (73% versus 58%). Jacob and colleagues postulated that SOD might cause recurrent episodes of pancreatitis even though SOM was normal and that pancreatic stent placement might further prevent further attacks.61 In a randomized study, 34 patients with IARP, normal pancreatic duct SOM, ERCP, secretin testing, and biliary crystals (probably best considered “true IARP”) were treated with pancreatic stents (n = 19, 5 to 7 Fr, with stents exchanged three times over a 1-year period) or conservative therapy (n = 15). During a 3-year follow-up, pancreatitis recurred in 53% of patients in the control group and only 11% of the stented patients (p <0.02). This study suggests that SOM may be an imperfect test, as patients may have SOD that is not detected at the time of SOM. However, long-term studies are needed to evaluate the outcome after removal of stents, and concerns remain regarding stent-induced ductal and parenchymal changes.62,63 Because of the concern of stent-induced injury to the pancreas, trial pancreatic duct stenting to predict the outcome from pancreatic sphincterotomy is not recommended.52
Wehrmann and colleagues evaluated the feasibility and effectiveness of botulinum toxin injection in patients with recurrent pancreatitis due to pancreatic sphincter hypertension.64 No side effects were noted in any of the 15 treated patients. Twelve patients (80%) remained asymptomatic at 3-month follow-up but 11 patients developed a relapse at a follow-up period of 6 +/– 2 months. These 11 patients underwent pancreatic or combined pancreatobiliary sphincterotomy with subsequent remission after a median follow-up of 15 months. This study showed that injection of botulinum toxin is safe, may be effective in the short term, and predicts the outcome from pancreatic sphincter ablation in patients having frequent episodes of pancreatitis, but the need for definitive sphincter ablation in the majority of patients limits its clinical use.
In summary, these data show that SOD is the most common cause of IARP when detailed endoscopic evaluation is performed. SOM should be considered the gold standard for diagnosing SOD. Complete sphincter evaluation requires manometric assessment of both the biliary and the pancreatic sphincters. Although the best endoscopic therapy of SOD warrants further investigation, there is mounting evidence that pancreatic sphincter ablation will be necessary in the majority of patients to achieve the best long-term results. However, at present, controversy persists as to the appropriateness of performing SOM in patients with IAP.65 In a recent editorial Tan and Sherman66 stated that although the above studies suggest that endoscopic therapy may benefit a majority of patients with IARP due to SOD, there are many limitations that need to be emphasized.
1. The majority of published studies are retrospective and suffer from incomplete follow-up, lack homogeneity of patient selection for therapy, and are not blinded or compared to an untreated group.
2. Uncontrolled prospective studies are prone to bias.
3. The length of follow-up of most studies is less than 3 years. Short follow-up may result in an underestimate of the true recurrence rates.
4. There are also differences in defining study outcome. Authors have used different outcomes, including documented recurrent pancreatitis; need for reintervention; or a grading system of no relief, good relief, and complete relief of symptoms.
5. There is a lack of a homogeneous population of IARP patients treated.
6. Variable interventions are used, with different studies performing biliary sphincterotomy, pancreatic sphincterotomy, or dual sphincterotomy with unclear reasons as to why the particular therapy was chosen. Completeness of sphincter ablation was also often not determined.
In the absence of well-conducted randomized controlled trials and long-term patient follow-up, many authorities consider the benefits of sphincter ablation therapy to be currently unproven.66–68
Pancreas Divisum
Pancreas divisum, the most common congenital variant of pancreatic duct anatomy, occurs when the dorsal and ventral ducts fail to fuse during the second month of gestation.69 With nonunion of the ducts, the major portion of the pancreatic exocrine juice drains into the duodenum via the dorsal duct and minor papilla. It has been proposed that a relative obstruction to pancreatic exocrine juice flow through the minor papilla with resultant pancreatic duct hypertension could precipitate recurrent pancreatitis in a subpopulation of pancreas divisum patients.70–72 A recent paper studying the frequency of pancreas divisum in patients with acute recurrent or chronic pancreatitis found that pancreas divisum was more common in patients with genetic mutations of the PRSS1, SPINK1, and CFTR genes (16%, 16%, and 47%, respectively). There was no difference in frequency of pancreas divisum in patients with idiopathic pancreatitis and no gene mutations (5%), control group (7%), and alcohol-induced pancreatitis (7%). The authors suggested that pancreatitis may be a cumulative effect of two cofactors rather than being caused by pancreas divisum alone.73 Whereas a few epidemiologic studies dispute the relation of pancreas divisum and pancreatitis, three lines of evidence favor this association: (1) histologic studies and pancreatograms have demonstrated features of chronic pancreatitis isolated to the dorsal pancreas, (2) numerous studies have shown a statistically significant higher prevalence of pancreas divisum in this patient population, and (3) numerous studies indicated symptom resolution by facilitating dorsal duct decompression endoscopically or surgically.69,74–76
The diagnosis of pancreas divisum is suspected at ERCP when only a small ventral duct system is visualized following contrast injection of the minor papilla. It is confirmed when the remainder of the pancreatic ductal system (dorsal duct) is visualized by injecting contrast into the minor papilla and there is no communication between the two ductal systems. The clinical presentation and response to therapy for incomplete pancreas divisum where there is a small filamentous communication between the ventral and dorsal ducts appears to be similar to complete pancreas divisum.3,77
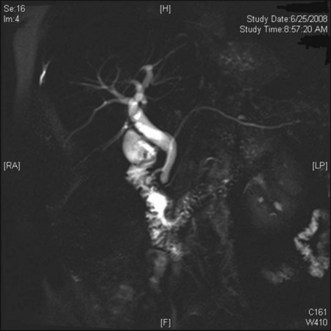
With the increasing availability of MRCP and improvement in pancreatic duct imaging with the use of secretin, S-MRCP is frequently used in the setting of IAP. Previous studies have reported a sensitivity and specificity of MRCP for detecting pancreas divisum (Fig. 49.3) of up to 100% (range of 36% to 100%).78–82
To assess the accuracy of MRCP in detecting pancreas divisum, Mosler and colleagues studied 146 patients undergoing MRCP with and without secretin followed by endoscopic retrograde pancreatography (ERP). Nineteen patients had pancreas divisum. The results showed that when S-MRCP was compared to ERP the overall sensitivity and specificity were 73% and 97%, respectively. The sensitivity and specificity improved to 83% and 99% in the subgroup of patients without chronic pancreatitis. The authors concluded that S-MRCP had a high specificity but only a modest sensitivity for pancreas divisum detection.83 In the setting of IARP, Mariani and colleagues showed that S-MRCP and ERP had similar detection rates of pancreas divisum in 8 of 44 (18.2%) and 7 of 43 (16.3%) patients.15
There are limited data on the role of EUS in diagnosing pancreas divisum.84,85 In the setting of IARP, Mariani and colleagues showed that S-EUS and ERP had similar detection rates of pancreas divisum in 6 of 44 (13.6%) and 7 of 43 (16.3%) patients.15
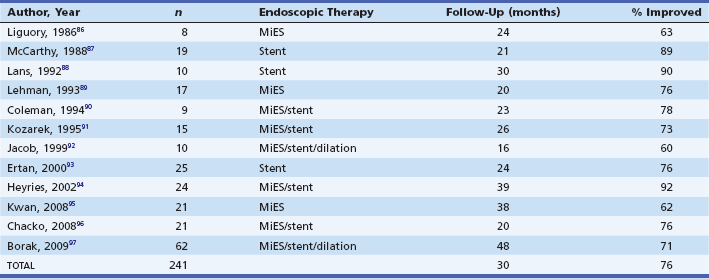
The aim of endoscopic therapy in symptomatic pancreas divisum patients is to alleviate the outflow of obstruction at the level of the minor papilla. The available endoscopic options include dilation, long-term dorsal duct stenting, minor papilla sphincterotomy, or a combination of therapies. Table 49.3 shows the outcome of endoscopic therapy in selected series.86–97 Overall, 76% of 241 patients had no further episodes of pancreatitis during a mean follow-up interval of 30 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree