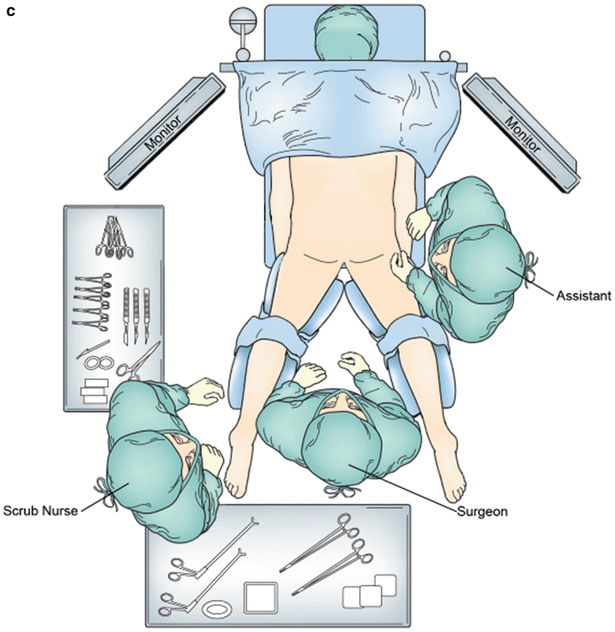
Fig. 28.1
Operative equipment and positioning. (a) Preoperative rectoscope. (b) Supine lithotomy position. (c) Prone position
Anesthesia
We recommend general anesthesia. The patient should be paralyzed, as any increase in the intraabdominal pressure will collapse the rectum and impede proper visualization of the lesion. These patients do not require high levels of analgesia during surgery.
TEM Equipment
The instrumentation required for TEM includes a 4-cm diameter rectoscope, in two different lengths (12 and 20 cm) (Fig. 28.2a). The choice of length depends on the site of the tumor. The rectoscope is secured to the operating table by a polyarticulated U-shaped holding system with a mechanical central clamp (Fig. 28.2b). The proximal part of the rectoscope contains a working attachment with four channels. Through one of these channels the vision system is inserted. This system incorporates a 10-mm 3-D stereoscopic telescope for the surgeon and a video-camera connection for the screen, which allows the rest of the team to follow the procedure. The other TEM instruments are introduced through the other three channels. These channels are sealed by rubber valves to prevent air leakage (Fig. 28.2b).
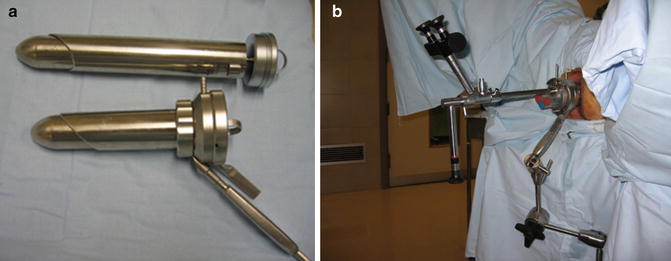

Fig. 28.2
TEM equipment. (a) Rectoscopes of two different lengths (12 and 20 cm). (b) TEM equipment in position with the U-shaped holding system
The TEM CO2 insufflator allows maintenance of a stable pneumorectum (10–12 mmHg) without the risk of excessive rectal distension. Its mechanism is based on continuous insufflation-aspiration in the rectum. The system allows the irrigation of the lens to obtain optimal vision via the TEM telescope [10].
The essential instruments for TEM all measure 5 mm in diameter: grasping forceps, ergonomic aspirator, a monopolar scalpel, needle-holder, clip-holder, and surgical scissors. We place two pedals on the ground: the left pedal is for the aspiration-irrigation system and the right pedal is for the bipolar electric scalpel.
TEO Equipment
The TEO equipment also includes a 4 cm diameter rectoscope. There are three different lengths (7.5, 15, and 22 cm). The choice of model depends on the site of the tumor. As in TEM, after draping and preparing the surgical field, the U-shaped holding system is mounted. The rectoscope is then introduced gently and attached to the holding system. The obturator of the rectoscope is withdrawn and the working attachment is secured in position. The silicon leaflet valves are checked to ensure that there is no air leak. The working attachment contains three more channels for instruments. The telescope guide measures 5 mm in diameter, compared with 10 mm in the case of TEM; this means that there is more space inside the rectoscope. The high-resolution digital camera is fitted to the 30° telescope. Then the cold light cable is inserted and the two tubes, one for insufflations and the other for smoke aspiration, are connected. The most recent models incorporate a new tube, which allows irrigation of the camera. In the new system, the smoke aspiration tube is fitted to the end of the rectoscope handle. On the ground there is only one pedal, which is for the bipolar electric scalpel. It is usually placed beneath the surgeon’s right foot. The high-definition screen is placed as directly in front of the surgeon as possible. The insufflation system is the same as the one used in any laparoscopy cart.
Standard Surgical Strategy
We work with a pneumorectum under a constant pressure of 10–12 mmHg. The rectal distension exposes the tumor and the rectal wall. If the patient is positioned correctly, the lesion should be in the lower part of the rectoscopic view. The first important maneuver before initiation of surgery is the mobilization of the rectoscope, ensuring that the rectal lumen is visible at all times. Then, we place the rectoscope over the lesion in order to gain access to the entire perimeter.
We place the rectoscope around 2 cm from the lesion. Before initiating dissection, we use the grasping forceps to check that we can reach the entire periphery of the lesion, or at least two-thirds of it. We assess its mobility by moving the adjacent mucosa. We stress that the TEM/TEO technique is dynamic: the rectoscope will be moved as many times as is necessary to achieve a position that facilitates the maneuver. On occasion, lesions in difficult locations can be accessed simply by repositioning the rectoscope.
We start the procedure by marking a dotted line with the electrocautery 10–15 mm around the tumor. We then open the mucosa along the dotted line and initiate the dissection of the lesion. The ultrasonic scalpel (UltraCision, Ethicon Endo-Surgery, Cincinnati, OH, USA) [34] is our instrument of choice for cutting the bowel wall. The curved tip of the ultrasound scalpel facilitates lateral dissection when working in parallel to the camera in a narrow field. With the setting in “low speed” the ultrasonic scalpel cuts through the rectal wall and mesorectal tissue without bleeding. The thin jaws allow a view of the tissue being sectioned, facilitating gradual advance. The grasping forceps are used to hold the healthy rectal mucosa, but never the tumor.
We begin the dissection at the most distal edge of the tumor in caudal-cephalo direction; that is, in the area closest to the rectoscope. Full-thickness excision of the rectal wall is performed in all cases, in search of the perirectal fat, known as “the yellow plane.” We continue laterally, then excise underneath and finish in the proximal area. The proximal area is usually the most difficult because it is impossible to lateralize the lesion, as we always work in parallel. After completing the excision, we irrigate with povidone-iodine solution diluted to 1 % using saline solution to induce cytolysis of any exfoliated cancer cells [10].
After excision, the surgeon or nurse attaches the specimen to a cork board and keeps the resection margins in place with pins to avoid retraction. The piece should also be oriented anatomically. In the case of broad margin resections, we indicate the correct position with respect to the specimen and also pin them in place.
The defect in the rectal wall must be sutured to avoid the risk of stenosing the rectal lumen (in large defects) and postoperative bleeding due to the traumatic effect of the tools. For suture, we use a 3-0 reabsorbable monofilament suture with an atraumatic cylindrical curved needle. A thread approximately 10 cm long is cut and inserted inside the rectoscope [10]. A silver clip or a LAPRA-TY (Suture Clip Applier, Ethicon Endo-Surgery, Cincinnati, OH, USA) is placed at the end of the suture to act as an anchor and avoid tying a knot (Fig. 28.3). A curved clip-holder is used, if possible, for suturing. Its ergonomic handle, which is easy to open and close, facilitates the maneuver. To introduce the suture into the rectoscope, first a rubber valve of the working channel is placed in the needle-holder (Fig. 28.3). Then the suture is held with the needle-holder as close to the needle as possible, and introduced into the rectoscope. With the suture inside the rectoscope, the needle is placed in the needle-holder with the help of the grasping forceps. The defect should be closed so as not to compromise the rectal lumen. The defect is closed from end to end with full-thickness stitches. The end of the suture is then secured with a second silver clip or Lapra-Ty. Occasionally, the rectoscope must be repositioned to achieve the optimal view. In large excisions it is helpful to begin suturing by placing one or two stitches in the center of the defect, so as to bring together its margins. We then perform two lateral running sutures. After finishing the suture, we again irrigate with a povidone-iodine solution diluted to 1 % with saline solution and the equipment is withdrawn.
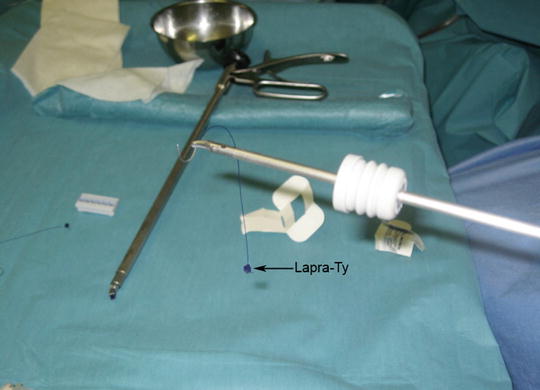

Fig. 28.3
The suture is held with the needle-holder as close to the end of the needle as possible and is then introduced into the rectoscope. A Lapra-Ty (Ethicon) has been placed on the end of the suture
In a comparative study of our experience with TEO and TEM, we found similar results with respect to surgical difficulty, postoperative morbidity, and quality of surgical resection, but lower economic cost with TEO [35].
Postoperative Management
We remove the Foley catheter in the operating theater or the recovery room. Patients are encouraged to ambulate and take a regular diet within 12 h of surgery, and they are usually discharged 2 days after surgery. Little postoperative analgesia is required: only nonsteroidal antiinflammatory drugs are typically needed. Thromboembolism prophylaxis is maintained for a month, as is standard practice in colorectal cancer surgery.
Technical Limitations of TEM
The distance of the upper edge of the lesion from the anal verge is of vital importance. Conventional endoanal excision is limited to lesions located at distances up to 7–8 cm. With TEM/TEO, the limits were set initially by the need to avoid penetrating into the peritoneal cavity. The risk of entering the peritoneal cavity was considered low in the setting of posterior tumors located up to 18–20 cm, and for anterior or lateral tumors located 15 cm or below. Today, perforation of the peritoneal cavity is not considered a contraindication for TEM/TEO [36]. There are no limits in terms of the location of the lesion (i.e., anterior, posterior, or lateral), as long as it can be technically removed. The real limit with respect to removal of the tumor is determined by the length of the rectoscope, and occasionally by other anatomical features such as the width of the rectosigmoidal junction or the rectal ampulla (below 10 cm), or a history of pelvic surgery that may impede the progression of the rectoscope. The limit for low lesions is the anal verge itself.
It is possible to excise adenomatous lesions that cover up to three quadrants of the circumference (10–12 cm). In fact, circumferential lesions, especially villous tumors, can sometimes be removed if they are not excessively long. The problem presented by large lesions is the need to suture the defect and the associated risk of stenosis.
Postoperative Morbidity and Mortality After TEM/TEO
Mortality rate among patients treated with TEM/TEO is low, and almost always occurs in patients with severe comorbid conditions who are treated for palliation. Postoperative morbidity ranges between 4 and 24 % [10, 37–40]. In contrast to TME, the vast majority of these complications are defined as minor; that is, they are complications that can be resolved with conservative treatment. In our series and in previous studies, the most frequent complication associated with TEM/TEO is postoperative bleeding (2–5 %). This bleeding tends to be self-limited, and only on very few occasions is colonoscopy with hemostatic intention or repeat TEM/TEO required.
Another minor complication is postoperative fever above 38 °C (5–10 %), which is well-tolerated by the patient and remits with conventional antipyretics within 24–48 h. This is usually self-limiting and does not require intervention. Suture dehiscence occurs in about 10–15 %, but this does not require change in postoperative management. Acute urinary retention is another rare complication in these patients, occurring in 2–7 %.
Among major complications, the most important is perineal sepsis due to rectal manipulation, principally in excision of the lower third of the rectum. It occurs in fewer than 2 % of cases. In this situation the initial treatment is antibiotic therapy with debridement. Only in exceptional cases is a terminal colostomy required to control the perineal sepsis. In our series of 523 cases, this was necessary in only two patients, both of whom were immunosuppressed.
A special situation is the risk of rectovaginal fistula in women, in the setting of tumors of the anterior wall of the rectum. In this setting, particular care is required when performing full-thickness wall excision, due to the risk of rectovaginal perforation. To avoid this complication, continuous digital vaginal examination should be performed. We have had three cases of rectovaginal fistula. Even though the perforation was sutured after TEM/TEO, the fistula persisted and required terminal colostomy and subsequent repair.
In our experience, if perforation into the peritoneal cavity is observed during surgery and can be sutured using TEM/TEO, it does not represent a postoperative complication. Prior to surgery, surgeons can gain a reasonable idea of the risk of perforation involved in resecting a particular lesion from the results of rectal MRI and by identifying the peritoneal reflection and its relation to the lesion [36, 41]. Perforations during surgery that pass unnoticed and are not properly sutured may cause intraabdominal sepsis and in these cases represent a major complication [10, 42–44].
References
1.
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–82.PubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








