Fig. 22.1
High-definition three-dimensional camera and visualization of pelvic structures. The female pelvic anatomy is demonstrated
A number of trials comparing robotic TME (RTME) and laparoscopic TME with respect to feasibility and long-term oncologic outcomes are forthcoming [14–16]. Recent review of outcomes associated with robotic-assisted, laparoscopic, and open surgical approaches to colorectal cancer indicates that the robotic approach is safe and feasible, demonstrating comparable short-term outcomes [17]. In a comparison of robotic-assisted versus open surgery for rectal cancer, Pigazzi et al. reported reduced complications and a reduction in postoperative pain allowing many patients undergoing robotic-assisted procedures to begin adjuvant treatment sooner [18]. Pigazzi et al. also reported no operative mortality, 7.3 % rate of conversion, 8.6 % rate of anastomotic leak, 13.1 lymph nodes retrieved, and 97.6 % negative circumferential resection margin in another series of 82 rectal cancer patients treated with RTME [19]. These outcomes are similar to other reports showing favorable results in patients undergoing RTME [19, 20, 28].
RTME is associated with similar short-term quality of life outcomes, similar rates of postoperative complications, equivalent short-term surrogate outcomes such as circumferential resection margin, number of harvested lymph nodes, and rates of clear distal margin but lower intraoperative conversion rates, compared to conventional laparoscopic TME [15, 16, 19, 21–23].
It should be noted that, while RTME facilitates pelvic dissection, it requires a longer operative time than open or traditional laparoscopic surgery. This is true even after the learning curve is reached, although the amount of operative time has been shown to decrease with surgeon experience [24]. Additionally, there is a relatively high learning curve of at least 15–20 cases [24–26]. The robotic system comes at a high price. This includes not only the cost of the robotic system itself (approximately $1.65–$2.5 million USD), but its maintenance [11, 27], raising significantly the costs per patient hospitalization compared to conventional laparoscopy (average $84,000 vs. $63,000, respectively).
Surgeon Requirements
As mentioned above, RTME requires a longer operative time. RTME technique is technically demanding, with a high associated learning curve [25, 26, 28]. Surgeons wishing to embrace RTME should meet the following criteria:
1.
Experience and comfort performing laparoscopic segmental colon resection without hand assistance.
2.
Robotic experience with inanimate models/simulators and animal and cadaveric models.
3.
A thorough understanding of the principles of TME and an adequate yearly volume of rectal procedures.
Patient Selection
We use robotic LAR for the patient who presents with nonsphincter-invading mid and low rectal cancer. However, surgeons who are just beginning to use the robotic system should offer this procedure more selectively. For a double-stapled anastomosis after LAR, the surgical distance between the lower edge of the tumor and the anorectal ring should be at least 1–2 cm. Thus, at the start of the learning curve, female patients with normal body mass index (BMI) and tumor located high above the anal verge are ideal patients.
Preoperative Considerations
Preoperative imaging of all patients with rectal cancer should involve chest X-ray, computed tomography (CT) scan of the abdomen and pelvis, and endorectal ultrasound (ERUS) or pelvic magnetic resonance imaging (MRI). Neoadjuvant chemoradiation (CRT) treatment should be offered to patients with locally advanced cancer, according to the surgeon’s preference and experience. A bowel preparation is recommended the day before surgery, as this makes intestinal manipulation easier.
Patient Positioning
We routinely place a large foam mat under the patient to prevent sliding during changes in the position of the operating bed. The upper chest is secured with a velcro strap and tested. After induction of general anesthesia, the patient is moved into a modified lithotomy position. A digital rectal examination is performed to confirm the location of the tumor. Routine rectal irrigation with water or an iodine-based solution is done. A urinary catheter is inserted once the patient is prepped and draped. The perineum is prepped in sterile fashion only if a transanal extraction and hand-sewn coloanal anastomosis are anticipated.
Totally Robotic Approach
Previously, we performed a hybrid/laparoscopic approach, but we now complete the procedure in a completely robotic manner. We complete the operation with one docking maneuver and minor patient repositioning. The entire intracorporeal procedure with transanal extraction and minimal incision will be described. It is important to note that this approach requires considerable expertise with robotic port placement and cart positioning, in order to avoid collisions with the robotic arm. This should become less problematic with the new generation robotic systems.
Port Placement
A Veress needle is inserted in the left subcostal region (Palmer’s point) and the abdomen is insufflated to 15 mmHg. A 12-mm camera (port C) is placed halfway between the pubis and xiphoid. As a general principle, robotic ports must be at least 8–10 cm apart to avoid collisions (Fig. 22.2). A 12-mm trocar (R1) is then placed roughly halfway between C and the right anterior superior iliac spine (ASIS), which corresponds to the midclavicular line (MCL). Great care must be taken to avoid the inferior epigastric vessels in this area. R1 will be the main stapling/clipping port for vessels, mesentery, and bowel; this site may also serve for the protective ileostomy. R1 is used by the surgeon’s right hand. A 5-mm laparoscopic port (L1) is placed 8–10 cm above R1 in the MCL. A robotic 8-mm trocar (R2) will be inserted in the same position on the left side. A third robotic port (R3a) is placed in the MCL, 8 cm above L1 (Fig. 22.2). The fourth robotic port (R3b) will be 8–10 cm more lateral to R2, usually just above the left ASIS. It is important to note that some variations in port set-up may be necessary depending on gender, body habitus, and tumor location. For example, in large male patients with low tumors, the four robotic ports will be shifted medially to prevent the robotic arms from hitting a narrow pelvic sidewall and to access the levator plane more easily.
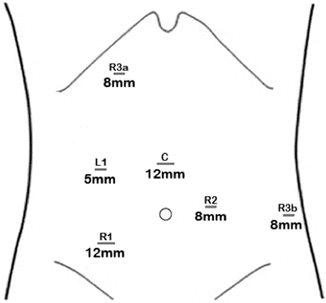

Fig. 22.2
Trocar positioning for robotic total mesorectal excision. All robotic ports must be 8–10 cm apart to avoid collisions
Mobilization of Splenic Flexure and Left Colon
For this portion of the procedure, the patient will be right side down and in moderate Trendelenburg position. The surgeon will control C, R1 and R3a, while the bedside assistant has L1. The surgeon can also utilize R2 to obtain better access to a high splenic flexure. A medial-to-lateral mobilization of the left and sigmoid colon is performed. The inferior mesenteric vein (IMV) is used as the initial anatomic landmark. To expose the IMV, the ligament of Treitz and attachments between the proximal jejunum and descending mesocolon may have to be divided, so the small bowel can be retracted towards the right upper quadrant (Fig. 22.3a).
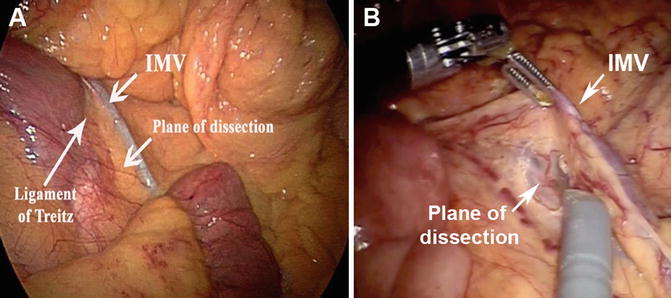

Fig. 22.3
Exposure and division of the inferior mesenteric vein (IMV). (a) View of the IMV and ligament of Treitz, with the plane of dissection indicated. (b) A clip has been placed on the IMV, and the dashed line shows the IMV traveling with the left colic artery. The medial-to-lateral dissection plane can be seen beneath the vessels
Next, the peritoneum just under the IMV is incised, and medial-to-lateral dissection begins. Dissection proceeds, with care taken to identify and preserve the ureter and gonadal vessels. To avoid traction injuries, we recommend early division of the IMV near the pancreas, where the IMV is traveling alone without a paired artery (Fig. 22.3b). More distally, the IMV runs parallel to the left colic artery (LCA). Therefore, the IMV/LCA pedicle should be followed inferiorly and freed from its posterior attachments to the aorta up to the origin of the IMA. The peritoneum over the sacral promontory just medial to the right common iliac vessels is incised, entering the areolar plane posterior to the superior rectal artery. By extending this dissection plane to the cephalad, the origin of the IMA is identified; the vessels create a characteristic T-shaped structure.
After identification of the ureter and gonadal vessels in the retroperitoneal plane, the IMA can be divided. The medial-to-lateral dissection is taken laterally toward the abdominal wall. The colon is then retracted medially; the peritoneum along the white line of Toldt is opened, completely freeing the descending and sigmoid colon. Next, the splenic flexure is taken down by opening the gastrocolic omentum just below the gastroepiploic vessels or dividing the avascular colo-epiploic attachments next to the bowel wall. The splenocolic ligament is then divided. We recommend using an energy-based vessel-sealing device for these steps. Lastly, the attachments of the body and tail of the pancreas to the colonic mesentery are carefully divided to obtain a full splenic flexure release.
The mesentery of the descending colon is then divided from the stump of the IMA towards the colon at the point of future division of the bowel, usually at the junction of the descending and sigmoid colon. The mesentery can be divided with an energy source or with several fires of a vascular stapler. We recommend dividing the marginal artery at this time to avoid tearing vessels during extraction, particularly if extraction of the specimen though the anus is anticipated.
Total Mesorectal Excision
After completing colonic mobilization, pelvic dissection can begin. The robotic arms are detached from the trocar, the patient is levelled, and a significant degree of Trendelenburg positioning is necessary to maintain the small intestine out of the pelvis. The robotic system should be redocked over the patient’s left hip, permitting access to the anus and perineum during the entire procedure (Fig. 22.4).
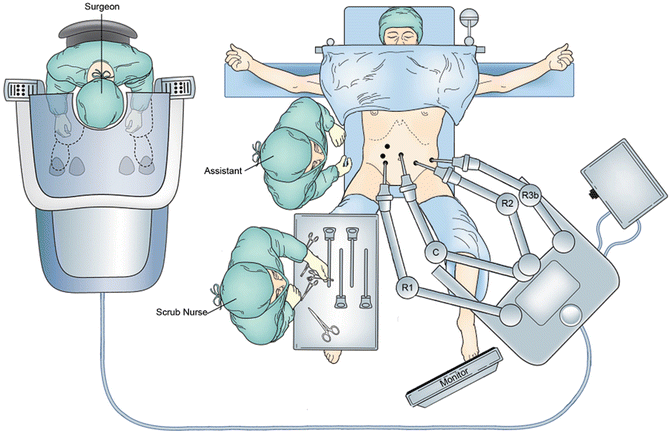

Fig. 22.4
The patient is repositioned for the total mesorectal excision. The robot is docked using a left hip approach
The camera arm with a zero degree telescope is placed in trocar C. Next, we attach a robotic trocar to arm 1 and “piggyback” this into the 12-mm R1 port. Arms 2 and 3 are docked to trocars R2 and R3b, respectively. With respect to instruments, we choose scissors for arm 1, a fenestrated bipolar grasper in arm 2, and a Prograsp grasper (Intuitive) in arm 3. The assistant remains on the right side, using ports L1 and R3a for suctioning and retraction of the rectum out of the pelvis.
With the assistant elevating the rectosigmoid junction, dissection begins posteriorly at the sacral promontory, entering the plane between the fascia propria of the rectum and the presacral fascia. Care must be taken to identify and preserve the hypogastric nerves bilaterally. The dissection is carried out almost exclusively with monopolar cautery, applied in short bursts with scissors to prevent excessive smoke accumulation and nerve injury. The TME proceeds along the areolar plane down to the rectococcygeal ligament. It is important to avoid grasping the mesorectum with robotic graspers because these instruments have considerable strength and can cause bleeding as well as injury to the fascia propria. We prefer to use the bipolar grasper in arm 2 as a retracting device.
Anteriorly, the peritoneal reflection is incised and the dissection is continued along the rectovaginal septum in women or the rectovesical/rectoprostatic fascia (Denonvillier’s fascia) in men. Arm 3 is very useful for retracting the bladder and other anterior structures as dissection proceeds distally. The articulation of the robotic scissor tips enables the surgeon to perform the dissection using ideal approach angles.
Laterally, dissection proceeds along the sidewalls medial to both ureters. Care must be taken to avoid injuring the autonomic pelvic plexus. The lateral stalks are controlled with bipolar cautery on arm 2 and are subsequently cut and divided.
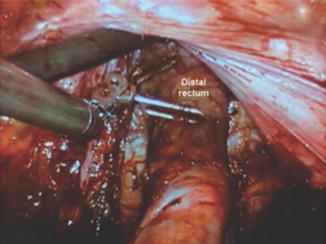
Dissection continues down to the pelvic floor, separating the fatty mesorectum from the levators. In preparation for rectal division, digital rectal examinations and brief endoscopic exams are performed regularly to ascertain the level of the tumor. The rectum is lifted off the levator muscle and prepared circumferentially for division (Fig. 22.5).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








