Thrombotic Microangiopathies: Introduction
The thrombotic microangiopathies (TMAs) are classified together due to their overlapping clinical and morphologic findings. Their major clinical manifestations include hemolytic anemia, microvascular thrombosis, and thrombocytopenia. Most TMAs have renal involvement with similar morphologic changes in the kidney reflecting the vasculopathy these lesions share. There are a number of underlying etiologic factors inducing TMA including systemic diseases, infection, and medications, which are summarized in Table 34–1.
Systemic disorders |
Thrombotic thrombocytopenic purpura |
Hemolytic uremic syndrome |
Antiphospholipid syndrome |
Systemic lupus erythematosus |
Neoplasms |
Malignant hypertension |
Infections |
Enteric pathogens |
Escherichia coli 0157:H7 |
Shigella spp. |
Salmonella spp. |
Campylobacter jejuni |
Yersinia |
HIV |
Streptococcus pneumonia |
Mycoplasma pneumoniae |
Legionella pneumophilia |
Coxsackie A and B virus |
Medications |
Calcineurin inhibitors |
Mitomycin C |
Gemcitabine |
Bleomycin |
Cisplatinum |
Cytosine arabinoside |
Daunorubicin |
Vincristine |
Ticlopidine |
Clopidogrel |
Quinine |
Miscellaneous |
Vaccinations |
Bone marrow transplantation |
Pregnancy |
TMAs originally were separated into the distinct disease entities of hemolytic uremic syndrome (HUS), thrombotic thrombocytopenic purpura (TTP), and scleroderma renal crisis according to their clinical features. In fact, HUS and TTP were suggested to be varying manifestations of a single disease. However, as the underlying pathogenetic factors are being elucidated, it is clear that the TMAs are distinct entities with specific etiologies and associated laboratory and clinical findings.
Hemolytic Uremic Syndrome
Historically, HUS and TTP were considered to be variable expressions of the same disease process. With the identification of etiologic roles for factor H in HUS and for ADAMTS 13 in TTP, these are now thought to be distinct disorders with overlapping clinical features. HUS may present in a diarrheal (D+) or a nondiarrheal (D−) form. Most cases of D+ occur in summer and autumn, and result from Shiga-like toxin producing bacteria, primarily Escherichia coli 0157:H7, although other organisms may be involved. Transmission has occurred via undercooked ground beef, contaminated water, and unpasteurized milk or apple cider, and epidemics may ensue. The Shiga-like toxin binds to colonic epithelium inducing inflammation and tissue injury thereby allowing the toxin to enter the circulation. The toxin then binds to vascular and renal tubular epithelial cell receptors resulting in endothelial injury, inflammation, thrombosis, and renal failure.
The D− form of HUS is less well understood. It is associated with a number of underlying clinical predisposing factors including genetic predisposition, medication use, nongastrointestinal infections, pregnancy, neoplasms, collagen-vascular diseases, systemic vasculitis, and bone marrow transplantation. Deficiency of prostacyclin PGI2 has been implicated in some cases of D− HUS, while other cases likely result from an abnormality of the coagulation cascade or the endothelial cell membrane creating a prothrombotic milieu. A more common atypical subset of HUS is characterized by complement dysregulation due to factor H or membrane cofactor abnormalities. Factor H regulates the alternative complement pathway and membrane cofactor is a transmembrane complement regulatory protein. Mutations leading to deficiency or loss of activity of these regulatory elements allow activation of complement with deposition on and injury to glomerular and vascular endothelial cells and platelet aggregation resulting in thrombosis. Factor H deficiency is inherited in an autosomal recessive manner and is associated with low C3 levels and earlier disease onset. Factor H functional abnormalities are inherited in an autosomal dominant mode and these patients have normal serum complement levels, a later disease onset, and often other initiating events or stimuli.
HUS classically presents as a triad consisting of microangiopathic hemolytic anemia, thrombocytopenia, and renal disease. The signs and symptoms overlap those of TTP, but in HUS there is a predominance of renal and hematologic features. D+ HUS is associated with watery to hemorrhagic diarrhea, and epidemics associated with ingestion of undercooked hamburger or unpasteurized milk or cider occur sporadically. The other systemic manifestations of the diarrheal and nondiarrheal forms are similar and include fever, severe hypertension, pulmonary edema, cerebral edema and seizures, congestive heart failure, and cardiac arrhythmias.
The classical features are microangiopathic hemolytic anemia and renal dysfunction, frequently presenting as acute renal failure in adults, and less commonly in children. D+ HUS is associated with infection, and the offending organism such as E coli 0157:H7, Salmonella, or Shigella may be identifiable in stool culture. Leukocytosis and fluid and electrolyte disturbances may be found.
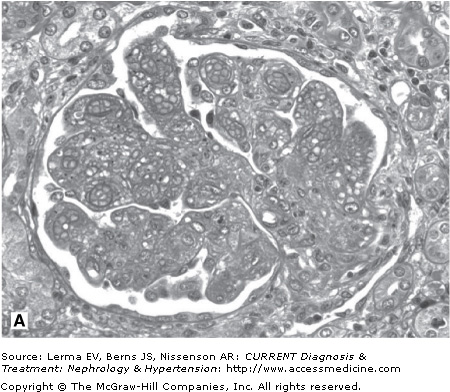
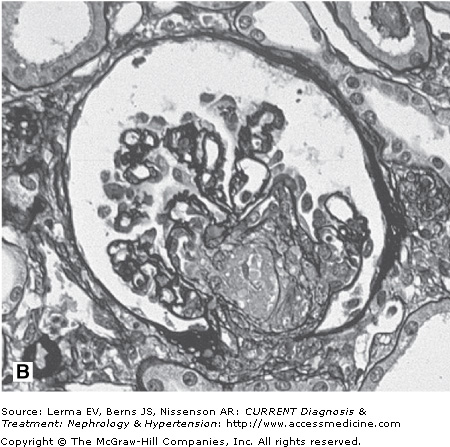
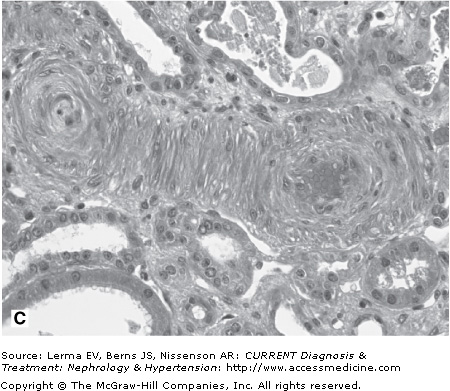
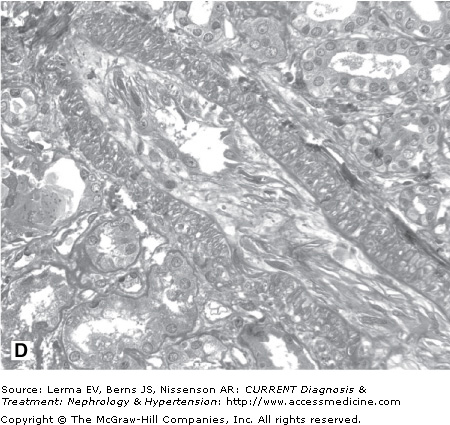
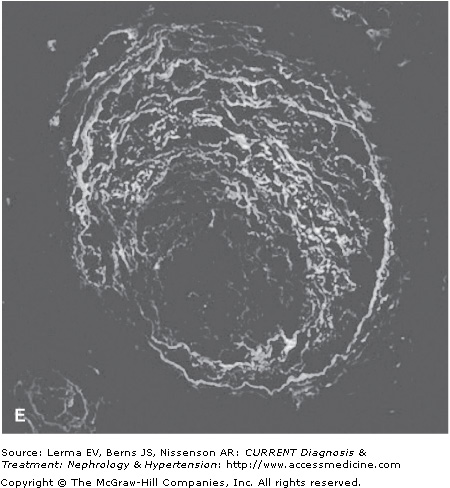
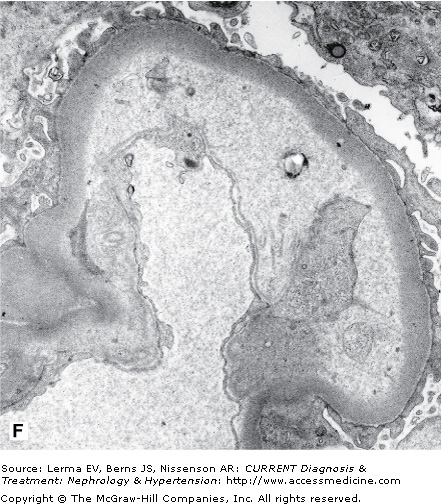
Renal biopsy findings are identical in the D+ and D± forms, as they are in all TMAs and are characterized by the accumulation of fibrin in the lumina and walls of arteries, arterioles, and glomerular capillaries (Figure 34–1). By light microscopy, fibrin and platelet thrombi are present in variable numbers of glomerular capillaries. Glomeruli may appear ischemic with wrinkled and partially collapsed capillaries or develop a lobular appearance with capillary wall double contours. Fibrin is in the walls and/or lumina of arterioles and, to a lesser extent, arteries, which also show muscular hypertrophy and mucoid intimal thickening, and endothelial cell swelling resulting in luminal narrowing. This may have a concentric or “onion skin” appearance. Immunofluorescence discloses fibrin in vascular walls and lumina, and glomerular capillaries without immune complexes. On ultrastructural examination, glomerular capillary walls have wide subendothelial zones containing flocculent electron-lucent and electron-dense material representing altered fibrin, which may contain trapped erythrocytes. Often there is a new layer of basement membrane material beneath the widened subendothelial zones accounting for the double contour appearance of capillaries. There are no electron-dense (immune complex) deposits. It has been suggested that patients with HUS may have more fibrin and erythrocytes with fewer platelets within intrarenal thrombi compared with TTP, and that HUS is more likely to have renal cortical necrosis than other TMAs. However, there are no definitive distinguishing features among any of the TMAs on renal biopsy.
Figure 34–1.
Renal findings in thrombotic microangiopathy. A: Glomerulus with a lobular configuration, mesangiolysis, and thrombosed capillary lumina (×400). B: Thrombosis of intraglomerular arteriole. The glomerulus is ischemic with wrinkled and partially collapsed capillary walls (×400). C: Arteriole showing muscular hypertrophy with an “onion skin” appearance, absent lumen, and focal thrombosis (×200). D: Artery with mucoid intimal thickening and swollen endothelial cells (×200). E: Immunofluorescence of an artery demonstrating fibrin throughout the thickened vascular intima. F: Electron micrograph of a glomerular capillary wall showing a markedly expanded subendothelial zone containing flocculent granular material (altered fibrin) with narrowing of the lumen.