Essentials of Diagnosis
- Acute onset of renal failure.
- Fever, skin rash, and peripheral eosinophilia in a minority of cases.
- Mild proteinuria, hematuria, and sterile pyuria; eosinophiluria detected by Hansel’s or Wright’s stain.
- Tubular dysfunction, manifested as glycosuria, aminoaciduria, potassium wasting, magnesium wasting.
Tubulointerstitial Diseases
Disease processes involving the part of the renal parenchyma that consists of the tubules and interstitium are primarily referred to as tubulointerstitial diseases. Tubulointerstitial diseases can be classified as acute or chronic and can present either as primary or secondary (to a systemic disease) processes. Histopathologically, the presentation can vary from a subtle accumulation of lymphocytes, monocytes, or macrophages in the interstitium or tubular atrophy or dilation to extensive interstitial fibrosis, which may be accompanied by glomerulosclerosis.
There are several ways in which injury to the tubulointerstitium can occur, and these can involve either immune-mediated or non-immune-mediated (direct toxicity) mechanisms.
Although not commonly performed, a renal biopsy still provides the most definitive means of diagnosis. From a practical standpoint, however, the diagnosis is usually based upon a combination of epidemiologic, clinical, and laboratory findings.
As an example, a simple urinalysis provides a gamut of information on tubulointerstitial diseases. Examination of the urinary sediment for red blood cells (RBCs), white blood cells (WBCs), and casts is particularly valuable. Dipstick analysis for protein is frequently positive, and when quantified, it is usually <2 g/day. Depending on which part of the tubules is injured, it is possible to observe glucosuria or aminoaciduria (proximal tubules), potassium or magnesium wasting (distal tubules), and salt wasting, as well as urinary concentrating defects or isosthenuria (medullary loop of Henle), manifested by polyuria. Normal anion gap metabolic acidosis is commonly observed, as in the various types of renal tubular acidoses.
Glomerular diseases are a distinct and separate pathologic entity, characterized by heavy or nephrotic-range proteinuria >3.5 g/day, RBC casts on urinalysis, as well as hypoalbuminemia. It is not uncommon, however, for both glomerular and tubulointerstitial disease to occur in a single patient.
Patients are often hypertensive at the time of presentation.
Acute tubulointerstitial nephritis (ATIN) usually presents as an acute rise in blood urea nitrogen (BUN) and creatinine values. The majority of affected patients typically present with nonspecific symptoms. The classic triad of fever, skin rash, and peripheral eosinophilia is seen in a minority of patients. Mild to moderate proteinuria, hematuria, and sterile pyuria are seen in the majority of cases. The occurrence of nephrotic-range proteinuria usually suggests concomitant glomerular disease. It must be noted that eosinophiluria is not specific for ATIN, as has also been demonstrated in other disease processes such as rapidly progressive glomerulonephritis and acute prostatitis as well as atheroembolic renal disease.
Under the microscope, the eosinophilic granules are more clearly demonstrated when Hansel’s stain is used, although, in some cases, Wright’s stain would suffice. Renal tubular acidosis features, such as glucosuria, aminoaciduria, as well as phosphaturia, indicate tubular injury.
Although the diagnosis of ATIN is usually suspected based on clinical grounds, the definitive diagnosis is established primarily by histopathologic features.
The causes of ATIN are conveniently classified into three general categories: Drug-induced ATIN, infection-associated ATIN, and systemic disorders, with immune-mediated mechanisms.
The patient’s history is crucial in determining the exact cause of ATIN because the majority of cases have been causally related to medication use, with approximately one-third of such cases being secondary to antibiotic usage.
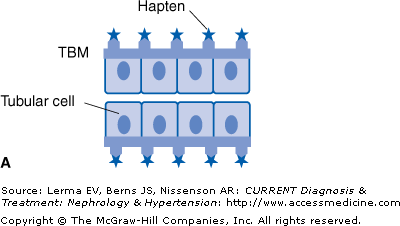
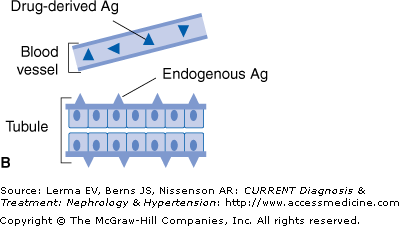
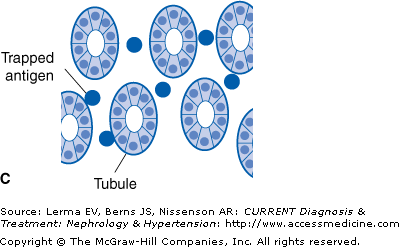
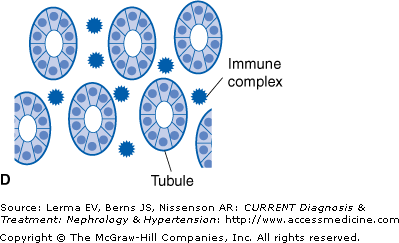
There are four different mechanisms by which a drug can induce ATIN (Figure 36–1).
Figure 36–1.
Mechanisms whereby a drug (or one of its metabolites) can induce acute interstitial nephritis (AIN). A: The drug can bind to a normal component of the tubular basement membrane (TBM) and act as a hapten. B: The drug can mimic an antigen normally present within the TBM or the interstitium and induce an immune response that will also be directed against this antigen. C: The drug can bind to the TBM or deposit within the interstitium and act as a planted (“trapped”) antigen. D: The drug can elicit the production of antibodies and become deposited in the interstitium as circulating immune complexes. (Adapted with permission from Rossert J: Kidney International 2001;60:804.)
The prototype agent for antibiotic-induced ATIN is methicillin. Because of this it is rarely if ever used in clinical practice today. In fact, it is no longer available in the United States. The list of medications causing ATIN continues to grow (Table 36–1).
Antimicrobial agents |
Acyclovir |
Ampicillin1,2 |
Amoxicillin |
Aztreonam |
Carbenicillin |
Cefaclor |
Cefamandole |
Cefazolin |
Cephalexin |
Cephalothin |
Cefoxitin |
Cefotaxime |
Cidofovir |
Ciprofloxacin |
Cloxacillin |
Colistin |
Cotrimoxazole2 |
Erythromycin |
Ethambutol |
Foscarnet |
Gentamicin |
Indinavir |
Interferon |
Isoniazid |
Lincomycin |
Methicillin2 |
Mezlocillin |
Minocycline |
Nafcillin |
Nitrofurantoin2 |
Norfloxacin |
Oxacillin2 |
Penicillin G 2 |
Piperacillin |
Polymyxin acid 2 |
Quinine |
Rifampicin2 |
Sulfonamides |
Teicoplanin |
Tetracycline |
Vancomycin |
NSAIDs including salicylates |
Alclofenac |
Azapropazone |
Aspirin |
Diclofenac |
Diflunisal2 |
Fenclofenac |
Fenoprofen |
Ibuprofen |
Indomethacin |
Ketoprofen |
Mefenamic acid |
Meloxicam |
Mesalazine (5-ASA) |
Naproxen |
Phenylbutazone |
Piroxicam |
Sulfasalazine |