Renal cell cancer (RCC) is the most common form of cancer of the kidney and accounts for approximately 44,000 cases per year in the United States. Historically, only immunotherapy showed activity in metastatic RCC. The improved survival and quality of life for patients with metastatic RCC over the last several years are direct results of advances made in understanding the development of RCC. Three targeted therapies—sunitinib, sorafenib, and temsirolimus—have been approved for use in the United States recently. Current research is aimed at developing new drugs and combining available drugs to improve upon the responses and survival seen with approved single agents.
Kidney and renal pelvis tumors represented approximately 3.5% of cancers and 2.3% of the deaths due to cancer in 2007 in the United States, where renal cell cancer (RCC) accounts for an estimated 85% of all kidney tumors. Therefore, in 2008, approximately 44,000 cases of RCC will be diagnosed with more than 11,000 RCC-related deaths in the United States. RCC can be categorized as conventional (clear cell, 70%–85% of cases), papillary (chromophil, 10%–15%), chromophobe (5%), collecting duct (<1%), and unclassified (3%–5%). There are also the recently described Xp11 translocation RCCs, all of which bear gene fusions involving the TFE3 transcription factor gene. They account for at least one third of pediatric tumors and are a rare cause of aggressive RCC in adults. Each subtype may have a sarcomatoid component. Since there is no evidence that sarcomatoid RCC develops de novo, it is not viewed as a subtype on its own. Instead it is a high-grade variant of the type from which it arose. When no antecedent subtype can be identified, sarcomatoid RCCs are categorized as unclassified RCCs.
At presentation, up to 30% of patients with RCC have metastatic disease and recurrence develops in approximately 40% of patients treated for localized disease. Hence a large proportion of RCC patients require systemic therapy. However, renal cell carcinoma is in general resistant to traditional cytotoxic chemotherapeutic agents and investigators have sought novel approaches to treatment. These approaches have until recently focused upon immune modulation. Cytokine therapy with either interferon or interleukin-2 (IL-2) was the standard of care for patients with metastatic RCC and allogeneic stem cell transplantation showed promise.
Improved understanding of the biology of RCC led to the study of so-called “targeted therapies.” As a result, over the last few years, therapeutic options have improved dramatically as broad-spectrum receptor tyrosine kinase inhibitors, vascular endothelial growth factor (VEGF) antibodies, and mammalian target of rapamycin (mTOR) inhibitors have shown impressive antitumor activity or prolonged survival relative to cytokines. Since 2005, two multitargeted tyrosine kinase inhibitors (TKIs) and one mTOR inhibitor have been approved by the US Food and Drug Administration (FDA) for the treatment of advanced RCC: sorafenib (FDA approved in December 2005), sunitinib (FDA approved in January 2006), and temsirolimus (FDA approved in May 2007). Since each histologic subtype of RCC is associated with distinct genetic and molecular alterations, the use of these signal transduction inhibitors in metastatic RCC highlights the fact that the unique biology associated with specific histologic subtypes of RCC therapy will likely be associated with different response rates with current and future agents. Therapy will probably eventually be tailored to the biology of the tumor, as is currently being done for other malignancies, such as breast cancer. This review summarizes the current state of systemic therapy for metastatic RCC.
Tumorigenesis of renal cell cancer
The improved survival and quality of life for patients with metastatic RCC over the last several years are direct results of advances in understanding the development of RCC. The genetics of RCC tumorigenesis has been reviewed elsewhere and is briefly summarized here with attention to the consequences for systemic therapy for RCC.
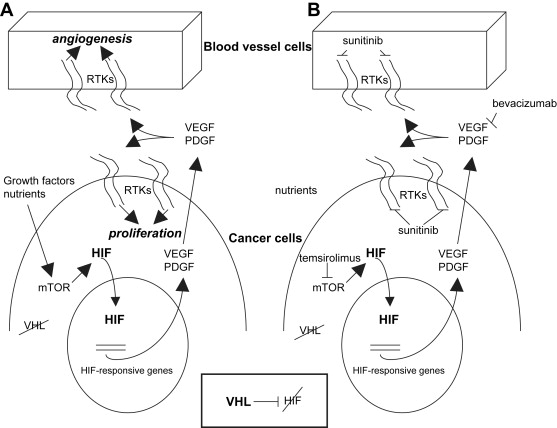
The von Hippel-Lindau (VHL) syndrome is an autosomal dominant disease characterized by the development of tumors in the cerebellum, spine, retina, inner ear, pancreas, adrenal glands, and kidneys. The kidney cancer in VHL syndrome is uniformly clear cell RCC, and affected individuals have hundreds of clear cell RCCs per kidney. The VHL tumor suppressor gene was identified in 1993. Both sporadic and inherited forms of clear cell RCC are strongly associated with mutations, deletions, or hypermethylations in the VHL gene ( VHL ), which inactivate the gene. The VHL protein functions as part of a multiprotein complex involved in targeting proteins for degradation by marking them with ubiquitin. Major targets that the VHL complex ubiquitinates are the transcription factors hypoxia-inducible factor 1α (HIF-1α) and hypoxia-inducible factor 2α (HIF-2α). Under normal oxygen conditions and with normal VHL function, HIFs are degraded. When hypoxia develops or if VHL is inactivated, HIF levels increase and HIF-dependent genes are transcribed ( Fig. 1 ). This leads to changes in expression of various proteins and constitutes the cellular response to hypoxia. HIF levels can also be regulated by growth factor and cell adhesion pathways, leading to activation of the Ras-Raf–mitogen-activated protein kinase pathway and the phosphatidylinositol 3-kinase-AKT-mTOR pathway. The HIF-dependent response is characterized by increased levels of VEGF, epidermal growth factor receptor (EGFR), platelet-derived growth factor (PDGF), glucose transporters (eg, GLUT-1), transforming growth factor-α (TGF-α, ligand for EGFR), and erythropoietin. In the context of a clear cell RCC, this results in stimulation of angiogenesis and tumor cell proliferation. Because VEGF has a central role during pathologic angiogenesis and restricted expression in healthy adults, a variety of therapeutic strategies aimed at blocking VEGF-induced signal transduction have been attempted. See Ferrara and colleagues for an excellent review of VEGF biology. Other RCC histologies are also associated with specific mutations. For example, type 1 papillary RCC is characterized by dysregulation or mutation in the tyrosine kinase domain of the c-Met oncogene.
Cytotoxic chemotherapy
Multiple reviews have summarized the poor results with traditional cytotoxic chemotherapeutic agents and hormonal therapies in metastatic RCC. Response rates were usually much less than 15%. Chemotherapeutic strategies for RCC were reviewed by Milowsky and Nanus 5 years ago in Urologic Clinics of North America . The investigators concluded that single-agent chemotherapy trials had been disappointing. They discussed the continued modest enthusiasm for combination chemotherapy, highlighting gemcitabine- and fluoropyrimidine-based regimens. However, two recently published trials of gemcitabine and capecitabine showed response rates of only 11% to 16% with significant toxicity. Stadler and colleagues concluded from their trial that the activity seen and degree of toxicity would not support the evaluation of gemcitabine plus capecitabine in a phase III trial. The combination of doxorubicin and gemcitabine has shown some activity in sarcomatoid and rapidly progressing RCC. In the study, the investigators collected the experience of two institutions (outside of a formal clinical trial) in treating patients with sarcomatoid and rapidly progressing RCC and found that, of 18 patients, 2 had a complete response and 5 had a partial response. A prospective cooperative group study with this regimen in patients with sarcomatoid features has completed enrollment and is currently being analyzed.
Cytotoxic chemotherapy
Multiple reviews have summarized the poor results with traditional cytotoxic chemotherapeutic agents and hormonal therapies in metastatic RCC. Response rates were usually much less than 15%. Chemotherapeutic strategies for RCC were reviewed by Milowsky and Nanus 5 years ago in Urologic Clinics of North America . The investigators concluded that single-agent chemotherapy trials had been disappointing. They discussed the continued modest enthusiasm for combination chemotherapy, highlighting gemcitabine- and fluoropyrimidine-based regimens. However, two recently published trials of gemcitabine and capecitabine showed response rates of only 11% to 16% with significant toxicity. Stadler and colleagues concluded from their trial that the activity seen and degree of toxicity would not support the evaluation of gemcitabine plus capecitabine in a phase III trial. The combination of doxorubicin and gemcitabine has shown some activity in sarcomatoid and rapidly progressing RCC. In the study, the investigators collected the experience of two institutions (outside of a formal clinical trial) in treating patients with sarcomatoid and rapidly progressing RCC and found that, of 18 patients, 2 had a complete response and 5 had a partial response. A prospective cooperative group study with this regimen in patients with sarcomatoid features has completed enrollment and is currently being analyzed.
Immunotherapy
Relapse of RCC many years after nephrectomy, prolonged disease stabilization without systemic treatment, and occasional spontaneous regressions suggested that host immune mechanisms might control tumor growth. This led to the study of immunotherapy for RCC. Interferon and IL-2 were reported to have antitumor activity in the 1980s and were the only proven therapy for metastatic RCC until recently. Immunotherapy for RCC has been the subject of a recently updated analysis by Coppin and colleagues for the Cochrane Collaboration. Combined data for a variety of immunotherapies gave an overall response rate of 12.4% (8.9% partial response and 3.5% complete response) compared with 2.4% in nonimmunotherapy control arms. High-dose IL-2 is associated with increased vascular permeability and requires inpatient monitoring, often in an intensive care unit. A limited number of centers now offer high-dose IL-2. It has been associated with a 4% incidence of treatment-related death and should be offered only to patients with normal cardiac, renal, and pulmonary function. However, response was poorly correlated with survival so that remission rate is not a good surrogate marker for survival for advanced RCC. High-dose IL-2 did not give better overall survival compared with low-dose IL-2 or to subcutaneous cytokine therapy, but may improve survival in the patients with bad prognostic factors based on subset analysis.
High-dose IL-2 was approved as treatment for RCC in the United States on the basis of phase II studies because of durable complete remissions seen in 5% to 7% of patients. The results of several studies indicate that interferon alpha (IFN-α) is superior to controls with a hazard ratio for death of 0.74. Doses ranging from 5 to 20 million units (MU) per day have been studied, although the optimal dose and schedule is not known. The addition of a variety of enhancers has failed to improve survival compared with IFN-α alone. Due to the limited availability of high-dose IL-2, its toxicity profile, and the survival associated with interferon, randomized studies of signaling inhibitors used interferon as the control arm. In addition, studies on cytokines have proven that surgery has a role in the treatment of metastatic RCC. In highly selected patients with metastases at diagnosis and minimal symptoms, cytoreductive nephrectomy before IFN-α therapy improves survival over IFN-α alone. Therefore, cytoreductive nephrectomy is a common part of systemic therapy for metastatic RCC. As a result, studies with signaling inhibitors (discussed below) have usually been performed in patients who have had prior nephrectomy.
Allogeneic stem cell transplantation
Other approaches to improve immunotherapy have been tried. It was hypothesized that graft-versus-tumor effects analogous to those seen in leukemias might occur in RCC. Childs and colleagues performed nonmyeloablative allogeneic peripheral-blood stem cell transplantation in 19 patients with suitable donors. The preparative regimen consisted of 60 mg/kg cyclophosphamide on day 7 and day 6 before transplantation, followed by 25 mg/m 2 fludarabine on each of the last 5 days before transplantation. Three patients (16%) had complete response and 7 had a partial response (37%) for an overall response rate of 53%. Two patients (11%) died of transplantation-related toxicity. In addition, consistent with a graft-versus-tumor effect, regression of metastases occurred a median of 129 days after transplantation, and often followed the withdrawal of cyclosporine and the establishment of complete donor–T-cell chimerism. These results were scientifically consistent with the initial hypothesis and were clinically extremely encouraging. A series of trials followed, including an intergroup trial that attempted to confirm these exciting results. Ueno and Childs recently summarized and reviewed the data from all trials. Most trials were small (7–25 patients) and used a variety of conditioning regimens and graft–versus–host disease prophylaxis. Response rates varied from 0% to 57%, and treatment-related mortality ranged from 0% to 40%. In the largest series, reported by Barkholt and colleagues, 124 patients were treated at 21 European centers with a variety of regimens. Transplant-related mortality was seen in 16% of patients. Out of 98 evaluable patients, 4 had complete response, 24 had partial response, and 24 had stable disease. Therefore, although autologous stem cell transplantation has not been optimized in this disease, it is clear that a graft-versus-tumor effect can be evoked against RCC. However, because the development of growth factor signaling inhibitors, the clinical necessity of this high-risk approach has lessened. Therefore, the risk/benefit profile of nonmyeloablative allogeneic peripheral-blood stem cell transplantation in RCC may relegate this approach to patients refractory to growth factor signaling inhibitors. Although allogeneic stem cell transplantation remains investigational in RCC, the improved treatments available may potentially diminish the suitability of patients who receive transplantation and lessen the chance of observing an antitumor effect.
Targeted therapies
VEGF antibodies, broad spectrum TKIs, and mTOR inhibitors are generally referred to as “targeted therapies.” However, most of the clinically useful TKIs would be better described as multitargeted. They are small molecules that bind to the ATP binding pocket of a group of evolutionarily related kinases with varying affinities ( Table 1 ). Some, such as sorafenib (which also inhibits raf), have an even broader spectrum of inhibition. The reason or reasons for the varying efficacy in the clinic with these agents is not clear. Therefore, although usually rationally designed, their use is therapy for RCC is somewhat empiric. Targeted therapies have recently been reviewed. Because the treatment of RCC is in rapid flux, we focus on targeted therapies in detail. Results of major randomized phase III trials are summarized in Table 2 .
| Kinase | Sunitinib (nmol/L) | Sorafenib (nmol/L) | Pazopanib (nmol/L) | Vatalanib (PTK787/ZK 222,584) (nmol/L) |
|---|---|---|---|---|
| VEGF receptor 1 | — | 26 | 10 | 77 |
| VEGF receptor 2 | 9–80 | 15–90 | 30 | 37 |
| VEGF receptor 3 | — | 20 | 47 | 660 |
| PDGF receptor α | — | — | 71 | — |
| PDGF receptor β | 2–8 | 57 | 84 | 580 |
| EGFR | > 20,000 | >10,000 | > 20,000 | — |
| Fibroblast growth factor receptor 1 | 830–2900 | 580 | 140 | — |
| Flt-3 and mutants | 10–300 | 33–58 | > 20,000 | — |
| Stem cell factor receptor (c-kit) | 1–10 | 68 | 74 | 730 |
| Ret | — | 47 | 2800 | — |
| Colony stimulating factor 1 receptor/c-fms | — | — | 146 | 1400 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








