Surgical Treatment of Stress Urinary Incontinence
Matthew D. Barber
Surgery for stress urinary incontinence (SUI) represents one of the most common indications for surgery in women. Approximately 4% of women will undergo surgery for SUI during their lifetime (1). An estimated 119,663 inpatient surgical procedures for SUI were performed in the United States in 2003, with the majority being performed for women age 45 to 64 (58,660) (2). The number of ambulatory procedures performed for SUI approximated 15,900 in 1996 and is certainly much higher today given the recent widespread adoption of minimally invasive sling procedures such as the tension-free vaginal tape (3).
HISTORICAL PERSPECTIVE
Over 1,000 surgical procedures for treating SUI have been described; however, only a small number have both withstood the test of time and held up to scientific scrutiny (Table 14.1). As of the writing of this chapter, only three techniques have consistently demonstrated superior efficacy for the treatment of SUI and are supported by level 1 evidence:
Retropubic colposuspensions, including the Burch colposuspension and the Marshall-Marchetti-Krantz procedure (MMK)
The traditional bladder-neck sling
The tension-free vaginal tape procedure
Several newer techniques show promise, such as the transobturator sling, but clinical trials evaluating their efficacy either have yet to be performed or are still ongoing.
The evolution of the surgical treatment of SUI largely occurred in the last century. In 1913, Howard Kelly first described his anterior plication stitch—a horizontal mattress stitch placed at the urethrovesical junction (UVJ) designed to narrow the patulous urethra and provide elevation of the bladder neck (4). The Kelly plication, along with later modifications by Kennedy (5), evolved into the modern-day anterior colporrhaphy. Because of its relative simplicity, low morbidity, and transvaginal approach, the anterior colporrhaphy became the primary treatment of SUI among gynecologists for much of the 20th century. After a number of studies demonstrated that the success rate for anterior colporrhaphy with Kelly plication was significantly less than that of retropubic colposuspensions or traditional slings, it fell out of favor for the treatment of SUI. While no longer an acceptable treatment for SUI, anterior colporrhaphy still remains an acceptable and commonly used technique for transvaginal correction of anterior vaginal prolapse.
The first suburethral sling procedure was described in 1907 by von Giordano using a gracilis muscle flap. In 1910, Goebel described detaching the pyramidalis muscle and suturing it beneath the urethra (6). Frangenheim modified the technique in 1914 by attaching a vertical strip of rectus fascia to the pyramidalis muscle (7). The final alteration of the initial Goebel-Frangenheim-Stoekel procedure included securing the pyramidalis muscle and the rectus fascia beneath the urethra after plication of the periurethral fascia (8). Later, in 1933, Price described the first sling constructed from fascia lata (9). In 1942, Aldridge described the rectus fascia
sling (10). He used two strips of rectus fascia sutured in the midline below the urethra via a separate vaginal incision. The fascial strips were brought down through the rectus muscle, behind the symphysis pubis, and united as a sling beneath the urethra at the UVJ. This provided a reliable cure for recurrent cases of SUI and served as the foundation for modern-day sling techniques. For most of the 20th century, sling procedures were used to treat patients with the most severe disease, those with recurrent incontinence and/or intrinsic sphincter deficiency, and were not used in the treatment of primary SUI. This is largely because sling procedures, as they were traditionally performed, were associated with an increased rate of voiding dysfunction and morbidity when compared to other prevailing techniques. It was not until the 1990s, after surgeons recognized the importance of “loosely tensioning” slings to minimize voiding dysfunction, that traditional sling procedures gained popularity as a first-line treatment for SUI.
sling (10). He used two strips of rectus fascia sutured in the midline below the urethra via a separate vaginal incision. The fascial strips were brought down through the rectus muscle, behind the symphysis pubis, and united as a sling beneath the urethra at the UVJ. This provided a reliable cure for recurrent cases of SUI and served as the foundation for modern-day sling techniques. For most of the 20th century, sling procedures were used to treat patients with the most severe disease, those with recurrent incontinence and/or intrinsic sphincter deficiency, and were not used in the treatment of primary SUI. This is largely because sling procedures, as they were traditionally performed, were associated with an increased rate of voiding dysfunction and morbidity when compared to other prevailing techniques. It was not until the 1990s, after surgeons recognized the importance of “loosely tensioning” slings to minimize voiding dysfunction, that traditional sling procedures gained popularity as a first-line treatment for SUI.
TABLE 14.1 Surgical Procedures for Stress Urinary Incontinence | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
The first retropubic operation for the treatment of SUI was described in 1949 by Marshall et al (11). In the mid-1940s, Victor Marshall, a urologist, began to develop an operation for treating voiding dysfunction that developed after rectal resection in men as a result of pronounced urethral hypermobility. He employed a suprapubic approach to suspend the bladder and bladder neck by placement of interrupted chromic catgut sutures to the periostium of the symphysis and posterior rectus sheath. Thereafter he collaborated with two gynecologists, Andrew Marchetti and Kermit Krantz, refining and modifying the procedure to treat urinary incontinence in women. Over the next several decades the MMK procedure became a standard for the treatment of SUI in women and is still used by some today.
John Burch described his retropubic colposuspension technique in 1961 after noting that when performing a MMK procedure the sutures in the periosteum of the pubic symphysis often pulled out (12). Burch identified Cooper’s ligament, the thick band of fibrous tissue running along the superior surface of the superior ramus of the pubic bone, as a more consistent point of attachment for the suspension sutures. In Burch’s original description of his operation, three sutures were placed in the periurethral tissues on either side and sutured to Cooper’s ligament. In 1976, Tanagho described his modification of the Burch procedure in which two sutures are placed in the anterior vaginal wall on each side, one at the midurethra and one at the UVJ, lateral enough to avoid damaging the urethral sphincteric mechanism (13). The sutures are passed through Cooper’s ligament and tied so that the urethra is preferentially elevated, but not compressed. He emphasized that the presence of a “suture gap” between the vaginal attachment and Cooper’s ligament was of no disadvantage and perhaps desirable. It is Tanagho’s modification of the Burch colposuspension that is most commonly performed today. While the available evidence suggests that the MMK procedure and the Burch colposuspension have similar efficacy, the Burch procedure is often preferred because it avoids the risk of osteitis pubis that is associated with the MMK.
The transvaginal needle suspension procedure was first described by Pereyra in 1959 (14). The needle urethropexy underwent more than 20 modifications in an attempt to improve the cure rates and minimize complications, including the Raz, Gittes, and Stamey procedures (15). Modifications involved various amounts of dissection and different anchoring tissue and materials. Although extremely popular in the 1980s and early 1990s, particularly among urologists, these procedures were largely abandoned after several comprehensive reviews and randomized trials demonstrated that they were significantly less effective than retropubic colposuspensions and traditional sling procedures (15).
The tension-free vaginal tape (TVT) procedure was introduced Ulmsten et al in 1996 and over the subsequent decade gained world-wide popularity (16). This operation introduced two new concepts to the mechanism of cure for slings: placement at the midurethra, and placement without tension (“tension-free”). The primary advantage that TVT offered over other surgical treatments for SUI, however, is that it could be performed on an outpatient basis. Often patients can void the day of surgery and be discharged home without a catheter. Several randomized trials and numerous cohort studies suggest that the TVT procedure has similar cure rates to the Burch colposuspension, with a quicker return to normal voiding and fewer postoperative complications (17, 18, 19). The success of the TVT has prompted the development of a number of similar minimally invasive midurethral slings with varying differences in sling material and surgical approach. To date, these “TVT-like” devices are largely unstudied.
The most recent innovation in the surgical management of SUI is the transobturator tape (TOT), which was first described by Delorme in 2001 (20). Like the TVT, this is a minimally invasive midurethral sling using a synthetic tape; however, it is placed using a transobturator approach rather than a retropubic one. The impetus for the development of this technique was to reduce the risk of bladder perforation and eliminate the rare but life-threatening complications of bowel perforation and major vascular injury that have been reported with TVT. Published data are limited regarding the relative efficacy and risk of complications with this new approach.
INDICATIONS FOR SURGERY
Surgery is indicated for the treatment of SUI when conservative treatments have failed to satisfactorily relieve the symptoms and the patient wishes further treatment in an effort to achieve continence (21). Prevailing opinion suggests that surgery should be delayed until childbearing is complete because the effect of subsequent pregnancy on continence surgery is unknown; however, the desire for future childbearing should not be considered an absolute contraindication (21,22).
Prior to surgery, the minimum evaluation should include a comprehensive history, physical examination, urinalysis, and measurement of postvoid residual volume. Stress incontinence should be objectively documented, with direct visualization of urine loss from the urethra with stress. Urethral hypermobility should be demonstrated with Q-tip testing or some similar method.
Urodynamics should be performed prior to surgery when the diagnosis is unclear or the patient is at high risk for treatment failure or complications. Not all patients with urinary incontinence require urodynamic testing prior to surgery, however. According to the AHCPR Urinary Incontinence Clinical Practice Guidelines, patients who lose urine only with physical exertion; have normal voiding habits (eight or fewer voiding episodes per day, two or fewer per night); have a normal neurologic examination and have no history of previous continence surgery or radical pelvic surgery; possess a hypermobile urethra and pliable vaginal wall on physical examination; have a normal postvoid residual volume and are not pregnant do not require urodynamics prior to continence surgery (23). These guidelines are based largely on expert opinion, however, and a considerable amount of research is required before evidence-based guidelines can be developed. Preoperative urodynamics should be strongly considered in patients with advanced age, a history of previous continence surgery, symptoms suggestive of detrusor overactivity or voiding dysfunction, an abnormal sacral neurologic examination, an elevated postvoid residual, or whenever the diagnosis of SUI is otherwise in question (21,23).
Factors that may negatively influence the results of SUI surgery include advancing age, obesity, a history of previous incontinence surgery, a nonmobile urethra, and preoperative detrusor overactivity (24). The evidence supporting these negative predictors is generally weak, however. As such, they should not be considered contraindications to continence surgery, but instead be used for patient counseling. Contraindications to SUI surgery include the presence of pure detrusor overactivity, an atonic bladder, or a neurogenic bladder. Also, patients who are otherwise at high risk for postoperative urinary retention who are unable or unwilling to perform self-catheterization may not be good candidates for SUI surgery.
Intrinsic Sphincter Deficiency
Subjects with severe urinary incontinence and urodynamic evidence of poor urethral sphincter function are said to have intrinsic sphincter deficiency (ISD), sometimes called type III incontinence or “low-pressure urethra.” Some authors have suggested that subjects with ISD are at risk for poor results after continence surgery, particularly after a retropubic colposuspension (24, 25, 26). They suggest that patients who demonstrate a low leak point pressure (less than 60 cm H2O) or low maximum urethral closure pressure (less than 20 cm H2O) are best served by a procedure such as a sling that is more obstructive. These findings are not consistent, however, with some authors finding no association between commonly used measures of urethral function and continence surgery success (27, 28, 29). Additionally, systematic reviews of the two most common measures of urethral sphincteric function, urethral pressure profilometry and leak point pressure measurement, have concluded that these tests are not well standardized and have poor reproducibility (30,31). In 2005, the World Health Organization’s Third International Consultation on Incontinence concluded that there is no consensus definition for ISD and there is currently no evidence that such a diagnosis influences the outcome of SUI surgery or should be used to choose the type of surgical treatment (24). In spite of this, many surgeons continue to use the results of urethral function testing in an attempt to provide prognostic information about the success of certain surgical procedures and to triage patients accordingly. A recent example is the suggestion by some that patients with ISD have a high failure rate after TOT procedures (32). Clearly, high-quality studies are needed to clarify the role of urethral function testing and the ISD diagnosis in the management of patients with SUI.
Mixed Urinary Incontinence
Approximately one third of patients with urodynamic stress incontinence have coexisting detrusor overactivity. These patients are said to have mixed urinary incontinence. There is some controversy about the best management for these patients. Studies have shown that patients with mixed urinary incontinence may have lower cure rates after surgery than those with pure SUI (33, 34, 35). Generally, 30% to 60% of women with mixed incontinence will have resolution of their urge incontinence after SUI surgery, with 5% to 10% developing worse urge incontinence and the remainder not changing (19,36,37). Unfortunately, attempts to use clinical or urodynamic data to predict who will improve and who will worsen have been unsuccessful (38). Most authors recommend that patients with mixed urinary incontinence undergo a trial of medical and behavioral therapy prior to considering surgery. Approximately one third of patients with mixed incontinence can be expected to become dry with conservative therapy alone (39). In those who have persistent bothersome incontinence after a trial of conservative therapy, surgery can be considered after appropriate patient counseling.
RETROPUBIC COLPOSUSPENSIONS
Retropubic colposuspensions are indicated for women with a diagnosis of urodynamic stress incontinence and a hypermobile urethra. They can be performed through an abdominal incision or laparoscopically. The Third International Consultation on Incontinence concluded that based on the currently available evidence, retropubic colposuspensions, particularly the open Burch colposuspension, “can be recommended as a procedure which is as effective as any other procedure for primary or secondary surgery with proven long-term success” in the treatment of SUI (24). The Burch colposuspension has historically been one of the most commonly performed operations for SUI, particularly among gynecologists. With the recent widespread adoption of minimally invasive slings, the popularity of the retropubic colposuspension has waned somewhat, with many surgeons reserving this procedure for instances where a laparotomy (or laparoscopy) is being performed for another indication (e.g., abdominal sacral colpopexy). No other continence operation has demonstrated greater efficacy or longer durability than the Burch colposuspension, however. As such, it should remain an important option in the surgical management of SUI.
Mechanism
SUI occurs when there is an unequal transmission of pressure between the bladder and urethra during stress such that the bladder pressure exceeds maximal urethral closure pressure (unaccompanied by a detrusor contraction), resulting in urine leakage. One commonly proposed theory holds that loss of urethrovesical support contributes to inefficient pressure transmission of the urethra because the urethra is displaced out of the abdominal cavity (40). Retropubic colposuspensions are designed to provide preferential elevation and support of the bladder neck by the placement of sutures in the
vagina near the urethra. This results in elevation of the hypermobile urethra back into an intra-abdominal position. Perhaps more importantly, it provides mechanical compression of the urethra against the stable, elevated anterior vaginal wall and/or the posterior-superior aspect of the symphysis pubis during episodes of increased abdominal pressure. The principal urodynamic change after these procedures is increased pressure transmission to the urethra, relative to the bladder, during elevations in intra-abdominal pressure (41,42). Resting urethral pressure and functional urethral length are unchanged, suggesting that the intrinsic function of the urethra is not altered appreciably by this type of surgery. Appropriate elevation of the bladder neck and urethra, accompanied by pressure transmission ratios near 100%, results in continence in most patients (42). Penttinen et al demonstrated a significant negative correlation between postoperative bladder neck mobility and pressure transmission ratios, suggesting that correction of the urethrovesical anatomic disorder eliminates the functional disorder and restores continence (43). Retropubic procedures, particularly the MMK, probably tend to overelevate and fix the urethra in a retropubic position. Hilton and Stanton found that pressure transmission profiles after successful Burch colposuspensions differed from those of continent control subjects, with pressure transmission ratios in the proximal half of the urethra significantly higher than 100% (44). This observation suggests that an additional mechanism that likely contributes to the success of these operations is partial outflow obstruction. Bump et al determined that patients with postoperative voiding abnormalities and detrusor instability after Burch colposuspensions had pressure transmission ratios significantly greater than 100%, supporting the hypothesis that obstruction, when excessive, also plays a role in postoperative voiding dysfunction and detrusor instability after these operations (41,42).
vagina near the urethra. This results in elevation of the hypermobile urethra back into an intra-abdominal position. Perhaps more importantly, it provides mechanical compression of the urethra against the stable, elevated anterior vaginal wall and/or the posterior-superior aspect of the symphysis pubis during episodes of increased abdominal pressure. The principal urodynamic change after these procedures is increased pressure transmission to the urethra, relative to the bladder, during elevations in intra-abdominal pressure (41,42). Resting urethral pressure and functional urethral length are unchanged, suggesting that the intrinsic function of the urethra is not altered appreciably by this type of surgery. Appropriate elevation of the bladder neck and urethra, accompanied by pressure transmission ratios near 100%, results in continence in most patients (42). Penttinen et al demonstrated a significant negative correlation between postoperative bladder neck mobility and pressure transmission ratios, suggesting that correction of the urethrovesical anatomic disorder eliminates the functional disorder and restores continence (43). Retropubic procedures, particularly the MMK, probably tend to overelevate and fix the urethra in a retropubic position. Hilton and Stanton found that pressure transmission profiles after successful Burch colposuspensions differed from those of continent control subjects, with pressure transmission ratios in the proximal half of the urethra significantly higher than 100% (44). This observation suggests that an additional mechanism that likely contributes to the success of these operations is partial outflow obstruction. Bump et al determined that patients with postoperative voiding abnormalities and detrusor instability after Burch colposuspensions had pressure transmission ratios significantly greater than 100%, supporting the hypothesis that obstruction, when excessive, also plays a role in postoperative voiding dysfunction and detrusor instability after these operations (41,42).
Access to the Retropubic Space
The patient is placed in modified lithotomy position using low leg holders such as Allen stirrups and is draped to allow both abdominal and vaginal access. The bladder is drained with a Foley catheter with a 10 cc or larger balloon. One perioperative intravenous dose of an appropriate antibiotic should be given as prophylaxis against infection. The abdomen may be entered through a transverse or vertical abdominal incision or laparoscopically. The laparoscopic approach will be discussed later in the chapter. While retropubic colposuspensions can be performed entirely retroperitoneally, some find that entering the peritoneal cavity and packing the bowel out of the pelvis often provides better visualization of the retropubic space. Entering the peritoneal cavity also allows for concurrent hysterectomy or additional abdominal prolapse repairs that may be necessary.
To access the retropubic space, the rectus abdominis muscles are separated in the midline and the underlying transversalis fascia is bluntly separated off the pubic symphysis. The retropubic space is developed using blunt dissection. The surgeon’s hand is placed along the underside of the pubic bone and the underlying bladder is displaced posteriorly. Sharp dissection is typically unnecessary in primary cases. Cooper’s ligament, the obturator neurovascular bundle, any accessory obturator vessels, and the lateral attachments of the vagina (arcus tendineus fascia pelvis) are identified. A fluffed-up gauze and a medium malleable retractor can be useful to retract the bladder medially to expose these lateral structures. The surgeon’s nondominant hand is placed into the vagina to elevate the paravaginal tissues and identify the urethra and bladder neck. Identification of the UVJ can be facilitated by gently placing traction on the Foley catheter and palpating the lower edge of the balloon. Adipose tissue is dissected off the anterior vaginal wall lateral to the urethra and UVJ using forceps, a peanut dissector, or some similar device. This dissection is facilitated by forcefully elevating the surgeon’s vaginal fingers until glistening white periurethral fascia and vaginal wall are seen. Dissection in the midline over the urethra and UVJ should be avoided to avoid trauma to the urethral sphincter mechanism. The retropubic space and paravaginal tissues are highly vascular, and careful attention and gentle dissection are required to avoid excessive bleeding. Hemostasis is achieved with hemoclips, cautery, or sutures.
If previous retropubic surgery has been performed, dense adhesions from the anterior bladder wall and urethra to the pubic symphysis are often present. These adhesions should be dissected sharply from the pubic bone until the anterior bladder wall, urethra, and vagina are free of adhesions and are mobile. If identification of the urethra or lower border of the bladder is difficult, one may perform a cystotomy, which, with a finger inside the bladder, helps to define the bladder’s lower limits for easier dissection, mobilization, and elevation.
Marshall-Marchetti-Krantz Procedure
The retropubic space is entered and the urethra and UVJ are exposed as described above. The surgeon’s
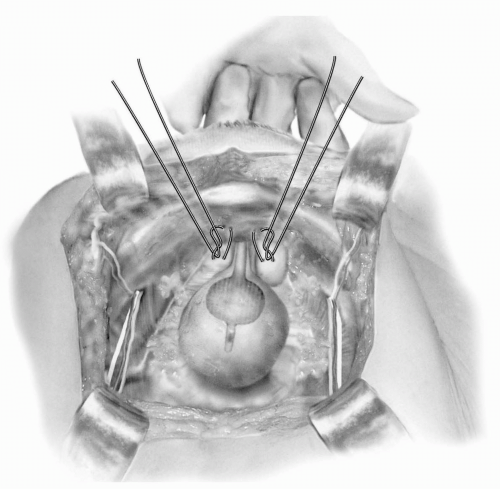
nondominant hand is placed in the vagina with the index and middle fingers placed on either side of the urethra to facilitate elevation of the urethra and UVJ. Permanent sutures are placed with the needle initially entering closest to the urethra and then coursing lateral perpendicular to the urethra to include almost the full thickness of the anterior vaginal wall. One to three pairs of sutures are placed on either side of the urethra, with the most proximal pair at the UVJ (Fig. 14.1). Sutures are generally placed much closer to the urethra during an MMK than is typical with a Burch colposuspension. All sutures are passed through the midline cartilage of the symphysis and tied. Hyperelevation of the urethra is avoided by tying the sutures so that there is sufficient space for the operator to easily place a finger between the pubic symphysis and urethra. Symmonds recommended performing a dome cystotomy when performing an MMK in order to allow directive visualization of the UVJ to facilitate appropriate placement of the proximal sutures (45). This is probably unnecessary with the use of routine intraoperative cystoscopy, however. Postoperatively, the bladder is drained with either a transurethral or suprapubic catheter until normal voiding occurs.
nondominant hand is placed in the vagina with the index and middle fingers placed on either side of the urethra to facilitate elevation of the urethra and UVJ. Permanent sutures are placed with the needle initially entering closest to the urethra and then coursing lateral perpendicular to the urethra to include almost the full thickness of the anterior vaginal wall. One to three pairs of sutures are placed on either side of the urethra, with the most proximal pair at the UVJ (Fig. 14.1). Sutures are generally placed much closer to the urethra during an MMK than is typical with a Burch colposuspension. All sutures are passed through the midline cartilage of the symphysis and tied. Hyperelevation of the urethra is avoided by tying the sutures so that there is sufficient space for the operator to easily place a finger between the pubic symphysis and urethra. Symmonds recommended performing a dome cystotomy when performing an MMK in order to allow directive visualization of the UVJ to facilitate appropriate placement of the proximal sutures (45). This is probably unnecessary with the use of routine intraoperative cystoscopy, however. Postoperatively, the bladder is drained with either a transurethral or suprapubic catheter until normal voiding occurs.
Burch Colposuspension
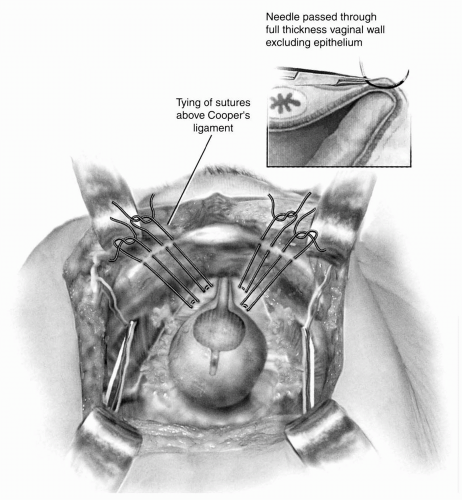
The retropubic space is entered, the urethra and UVJ are identified, and the periurethral anterior vaginal wall is cleared of all fat as described above. Two permanent sutures are placed on each side of the urethra through the anterior vaginal wall using double bites for each suture. Sutures should be placed almost full thickness through the anterior vaginal wall with the needle parallel to the urethra. The proximal suture is placed approximately 2 cm lateral to the UVJ. The distal suture is placed 2 cm lateral to the mid-urethra. The index and middle fingers of the vaginal hand are used to elevate the anterior vaginal wall on either side of the urethra during placement of the sutures. Alternatively, Allis clamps can be used to grasp and elevate the periurethral tissue at the proximal and distal suture sites and the sutures can be placed underneath the clamp in order to avoid an inadvertent needle injury of the vaginal fingers. On each side, after the two sutures are placed, they are passed through Cooper’s (pectineal) ligament so that all four suture ends exit above the ligament (Fig. 14.2). The sutures are tied so that there is a small amount of preferential elevation to the urethra while allowing two fingers to easily fit between the pubic bone and the urethra. A suture bridging between anterior vaginal wall and Cooper’s ligament is desired in order to prevent compression or hyperelevation of the urethra. As noted previously, this area is extremely vascular, and visible vessels should be avoided if possible. When excessive bleeding occurs, it can be controlled by direct pressure, sutures, cautery, or hemoclips. Less severe bleeding usually stops with direct pressure and after tying the Burch sutures. At the end of the procedure, cystoscopy should be performed to document the absence of intravesical sutures. Closed-suction drainage of the retropubic space is rarely indicated. Postoperatively, the bladder is drained with either a transurethral or suprapubic catheter until normal voiding occurs.
Laparoscopic Burch Colposuspension
Advances in minimally invasive techniques in the 1990s allowed for the development of the laparoscopic retropubic colposuspension. The potential advantages of the laparoscopic approach over the open approach include improved visualization of the retropubic space, shortened hospital stay, decreased postoperative pain, faster recovery, and improved cosmesis. Disadvantages of the laparoscopic approach include a steep learning curve in acquiring suturing skills, technical difficulty of retroperitoneal dissection, increased operating time early in the surgeon’s experience, and potentially greater costs related to longer operating time and use of disposable surgical instruments. Although various modifications have been described in an attempt to overcome the technical difficulty associated with laparoscopic suturing, many experts agree that laparoscopic retropubic colposuspensions should be performed in a manner identical to that of the open procedure, with the only difference being that of access to the retropubic space (46, 47, 48). The use of mesh, staples, bone anchors, or similar devices cannot be recommended.
As with the open procedure, the patient is placed in modified lithotomy position using low leg holders such as Allen stirrups and is draped to allow both abdominal and vaginal access. A three-way Foley catheter with a 20- to 30-mL balloon is attached to continuous drainage, and the irrigation port is connected to sterile water or saline. For the transperitoneal approach, a 5- or 10-mm trocar and laparoscope are placed through a standard infraumbilical incision. Two additional lateral trocars are placed: a 5/12-mm disposable trocar with reducer in the right lower quadrant (if knot-tying from the right) lateral to the right inferior epigastric vessels and a reusable 5-mm port or an additional 5/12-mm disposable trocar with reducer in the left lower quadrant lateral to the left inferior epigastric vessels. Trocars are placed lateral to the rectus muscle, approximately 3 cm medial to and above the anterior superior iliac spine. The retropubic space is developed by first identifying the two medial umbilical folds (the peritoneum overlying the obliterated umbilical arteries). These serve as lateral landmarks of dissection for transperitoneal entry into the retropubic space. The upper margin of the dome of the bladder is located several centimeters above the pubic symphysis. This can be easily visualized by filling the bladder with 300 cc of fluid via the three-way Foley. After emptying the bladder, the peritoneum is incised 2 cm above the bladder dome in between the medial umbilical folds. The retropubic space is developed with blunt dissection and important landmarks are identified as described earlier for the open procedure.
Although used less commonly, some prefer to access the retropubic space using an extraperitoneal approach. For this approach an infraumbilical incision is made with dissection to the preperitoneal space. The dissection is carried caudal into the retropubic space using a balloon dilator or similar technique. Once the retropubic space is entered, CO2 is insufflated to develop a “pneumo-Retzius,” additional trocars are placed, and the remainder of the procedure is performed similar to the transperitoneal approach.
Using laparoscopic needle drivers, 0- or 2-0 permanent sutures with an SH or CT-2 needle are placed at the bladder neck and midurethra on each side and then brought through Cooper’s ligaments, similar to the open procedure. Extracorporeal knot-tying is preferred because of technical facility and the ability to hold more tension on the suture. Thirty-six-inch or 48-inch sutures are necessary to facilitate extracorporeal knot-tying. At the completion of the procedure, cystoscopy is performed to assess the integrity of the bladder. Postoperatively, the bladder is drained with either a transurethral or suprapubic catheter until normal voiding occurs. Peritoneum of the retropubic space may be left open or closed according to surgeon preference. Generally patients can be discharged on the day of surgery after this procedure.
Adjuvant Procedures
Although hysterectomy is frequently performed at the time of SUI surgery, prospective studies, including clinical trials, demonstrate that the addition of a hysterectomy at the time of retropubic colposuspension does not improve SUI cure rates. Langer et al randomized 45 subjects to Burch culposuspension alone or colposuspension plus abdominal hysterectomy and cul-de-sac obliteration (49). Six months after surgery the objective (urodynamic) cure rates were not significantly different between groups (95.5% vs. 95.7% respectively). In 2001, Meltomaa et al reported their results from a prospective study evaluating morbidity and long-term subjective outcomes between Burch colposuspension alone and Burch with abdominal hysterectomy (50). There was no difference
in subjective outcomes up to 5 years after surgery. Complications were higher in the Burchplus-hysterectomy group (46.2%) than in the group who received a Burch alone (29.2%). These studies support the conclusion that hysterectomy should not be routinely performed at the time of a retropubic colposuspension unless there is a clear indication for the hysterectomy other than SUI.
in subjective outcomes up to 5 years after surgery. Complications were higher in the Burchplus-hysterectomy group (46.2%) than in the group who received a Burch alone (29.2%). These studies support the conclusion that hysterectomy should not be routinely performed at the time of a retropubic colposuspension unless there is a clear indication for the hysterectomy other than SUI.
Pelvic organ prolapse, particularly apical and posterior vaginal wall prolapse, has been reported to occur in 22.1% of women (range 9.5% to 38.2%) after a Burch colposuspension (24). Most are asymptomatic, however, and less than 5% request subsequent reconstructive surgery (24). Whether these findings are the result of an increased propensity for patients with SUI to develop future prolapse or are a direct result of the Burch procedure itself is largely unknown; however, pelvic organ prolapse appears to be more common after Burch colposuspension than after anterior colporrhaphy and sling procedures (19). In a randomized trial comparing the open Burch colposuspension to the TVT, anterior vaginal wall prolapse was more common in the TVT group but enterocele and apical prolapse were more common in the Burch group 2 years after surgery (17). In order to reduce the risk of subsequent prolapse, many authors suggest that a prophylactic culdesac obliteration procedure such as a uterosacral plication, Moschcowitz procedure, or Halban’s culdoplasty be performed at the time of retropubic colposuspension whenever possible. Although frequently advocated, the efficacy of this prophylactic maneuver is unstudied. At a minimum, patients who receive a retropubic colposuspension should be assessed for concurrent vaginal support defects at the time of their surgery and, when present, these should be corrected.
Outcome
A Cochrane Collaboration review of retropubic colposuspensions in 2005 identified 39 randomized clinical trials involving a total of 3,301 women, making retropubic colposuspension the most studied surgery for SUI in terms of level 1 evidence (19). The available evidence indicates that open retropubic colposuspension is an effective treatment for SUI, especially in the long term (19). Within the first year of treatment, the overall continence rate is approximately 85% to 90%. After 5 years, approximately 70% of patients can expect to be dry.
Both the Burch colposuspension and the MMK procedure appear to be durable, with only modest declines in efficacy over 10 to 20 years. Langer et al followed 127 women who received a Burch colposuspension for an average of 12.4 years and reported an objective cure rate of 93.7% (51). All failures in this study occurred within 1 year of the operation. Alcalay et al followed a cohort of 109 women after a Burch colposuspension for 10 to 20 years and found that the cure rate was time-dependent, with an initial decline for 10 to 12 years and a plateau of 69% thereafter (52). McDuffie et al found that the efficacy of the MMK procedure declined from 90% at 1 year to 75% at 15 years (53).
Retropubic colposuspensions have demonstrated a lower subjective failure rate than anterior colporrhaphy in six randomized trials with a relative risk (RR) of failure of 0.43 (95% CI 0.32 to 0.57) at 1 to 5 years after surgery and a RR of 0.49 (95% CI 0.32 to 0.75) at greater than 5 years (19). Similarly, retropubic procedures have demonstrated a lower failure rate than needle procedures in seven clinical trials, particularly after the first year postsurgery (RR 0.48; 95% CI 0.33 to 0.71) (19). The retropubic colposuspension has been compared to the paravaginal defect repair in a single randomized trial. Colombo et al found that after 6 months of follow-up, the objective cure rate of the Burch procedure was 100% compared to only 72% for those undergoing a paravaginal repair (54).
Retropubic colposuspensions have been compared to traditional sling procedures in five trials, and in each there was no significant difference between the two techniques, regardless of whether the procedure was a primary or secondary operation (55). However, these five trials all had small sample sizes (n = 22 to 72), limiting their ability to detect even large differences between the two treatments. The NIH-sponsored Urinary Incontinence Treatment Group (UITN) has recently completed the Stress Incontinence Surgical Treatment Efficacy Results (SISTEr) Trial, a randomized trial of autologous rectus fascia sling versus Burch colposuspension for the treatment of SUI with urethral hypermobility (56). This trial enrolled over 650 subjects from nine centers and followed them for a minimum of 2 years. As of the writing of this chapter, the results of this trial have not been reported. The size, quality, and scope of this trial should provide significant insight into the relative efficacy of these two “gold standard” operations.
Ward et al performed a large multicenter randomized trial comparing open Burch colposuspension to TVT for urodynamic SUI (17,57). They found no significant difference in objective or subjective cure rates of these two procedures. Six months after surgery the objective cure rate, defined as a negative 1-hour pad test and negative
stress test on urodynamics, was 57% for the Burch group and 66% for the TVT group (p = 0.10) when the authors considered those subjects who withdrew or were lost to follow-up as treatment failures (57). When the authors analyzed their data at 2 years and ignored subject withdrawals, the objective cure rate (negative 1-hour pad test) was 80% for colposuspension and 81% for TVT (17).
stress test on urodynamics, was 57% for the Burch group and 66% for the TVT group (p = 0.10) when the authors considered those subjects who withdrew or were lost to follow-up as treatment failures (57). When the authors analyzed their data at 2 years and ignored subject withdrawals, the objective cure rate (negative 1-hour pad test) was 80% for colposuspension and 81% for TVT (17).
Most studies comparing the Burch procedure to the MMK are retrospective and demonstrate similar cure rates for the two procedures. Only two randomized trials have compared these two procedures directly. Colombo et al randomized 80 women to either Burch colposuspension or MMK and followed them for 2 to 7 years (58). Differences in cure rates were not statistically significant between the two groups, with a subjective cure rate of 92% in those who received a Burch and 85% for those who received an MMK and objective cure rates of 80% and 60%, respectively (58). Burch colposuspension was associated with shorter hospital stay (mean difference of 1 day) and later resumption of voiding than the MMK (mean difference of 8 days) in this trial, however. Liapas et al randomized 170 women with SUI to receive a Burch colposuspension, a MMK procedure, or an anterior colporrhaphy and followed them for up to 5 years. The Burch procedure had a significantly greater subjective cure rate (88%) than the MMK (67%) or the anterior colporrhaphy (52%) (59). The results of these two trials and the risk of osteitis pubis that is uniquely associated with the MMK suggest that the Burch colposuspension should be the retropubic procedure of choice for SUI.
In 2003, the Cochrane Incontinence Group published a systematic review on laparoscopic retropubic colposuspension (60). They identified five randomized trials comparing laparoscopic to open colposuspension. A meta-analysis of these trials found similar subjective cure rates between the two approaches, ranging from 85% to 96% in the laparoscopic group and 85% to 100% in the open group 6 to 18 months after surgery (60). In contrast, objective cure (stress test at urodynamics) favored open colposuspension over the laparoscopic approach (RR 2.30, 95% CI 1.06 to 4.99) (60). One trial of subjects undergoing laparoscopic colposuspension demonstrated that two sutures on each side of the urethra resulted in a significantly higher cure rate than one suture (48). Notably, the trials included in this review have small sample sizes and short follow-up and are of relatively poor quality. Three of the five studies have only been published as abstracts. These weaknesses limit the strength of the review’s conclusions.
Since the publication of the Cochrane review, Smith et al presented the results of a large multicenter trial comparing open to laparoscopic Burch colposuspension (61). Two hundred ninety-one subjects were randomized from one of six centers in the United Kingdom and followed for 2 years. Two years after surgery, the objective cure rate (negative pad test) was similar for the two procedures (79% for the laparoscopic colposuspension vs. 70% for the open procedure) (61). The proportion of subjects reporting that they have “never leaked” since their procedure was 55% in the laparoscopic group and 53% for the open group. Laparoscopic Burch colposuspension was associated with decreased postoperative pain, decreased infectious morbidity, and greater cost than open Burch colposuspension.
In 2004, Paraiso et al randomized 72 women with SUI to receive a laparoscopic Burch colposuspension or a TVT (62). One year after surgery there was a greater rate of urodynamic stress incontinence in the laparoscopic Burch colposuspension group than the TVT group: 18.8% versus 3.2% (RR 1.19, 95% CI 1.00 to 1.42). Additionally, the time to development of recurrent urinary incontinence symptoms was earlier after laparoscopic Burch than with TVT.
Thus, while laparoscopic Burch colposuspension provides a minimally invasive alternative to its open counterpart, its role in the current environment of minimally invasive slings is unclear.
Complications
In general, the rate of perioperative complications associated with retropubic colposuspensions is low. In a review of 2,712 MMK procedures, Mainprize and Drutz noted a lower urinary tract injury rate of 1.6%, a wound complication rate of 5.5%, and a fistula rate of 0.3% (63). Similarly, Kenton et al noted that after Burch colposuspension lower urinary tract injuries were uncommon (less than 1%), while incisional complications were the most frequent perioperative complication (3%) (64). While laparoscopic colposuspension is associated with a shorter hospital stay and less blood loss than open colposuspension, the Cochrane review noted a longer operating time and a trend toward higher complication rates with the laparoscopic approach (60). Smith et al noted that laparoscopic colposuspension was associated with a lower infectious morbidity rate than open colposuspension, but otherwise there was no difference in complications between the two approaches (61). While nerve injury appears to be uncommon after retropubic colposuspension, Galloway et al have described the “post-colposus-pension
syndrome” in which women develop pain in one or both ilioinguinal regions following colposuspension (65). Demirci reported the occurrence of groin or suprapubic pain in 15 of 200 women (6.8%) after Burch colposuspension (66).
syndrome” in which women develop pain in one or both ilioinguinal regions following colposuspension (65). Demirci reported the occurrence of groin or suprapubic pain in 15 of 200 women (6.8%) after Burch colposuspension (66).
The most common long-term complication after retropubic colposuspension is de novo urge incontinence, which occurs in 5% to 27% of cases (19,24). Transient voiding dysfunction occurs in 6% to 37% of subjects after a Burch colposuspension, depending upon how it is defined (24). Voiding dysfunction that persists beyond 6 weeks after surgery is uncommon, however. Viereck et al reported persistent voiding difficulties in 3.5% of 310 women who underwent a Burch colposuspension with a mean follow-up of 36 months (67). Risk factors for prolonged voiding after a Burch colposuspension include advanced age, previous incontinence surgery, increased first sensation to void on preoperative urodynamics, high postvoid residual volume preoperatively, and postoperative cystitis (68).
Generally, overactive bladder symptoms and voiding dysfunction that occur after a retropubic colposuspension can be managed conservatively or are self-limiting. When these symptoms are refractory to behavioral or medical management, a urethrolysis performed either retropubically or vaginally may provide relief.
Osteitis pubis occurs after 0.74% to 2.5% of MMK procedures but is rare after Burch colposuspension (24). Patients with osteitis pubis typically present 2 to 12 weeks after surgery with suprapubic pain radiating to the thighs that is exacerbated by walking or abduction of the thighs, along with marked tenderness of the pubic symphysis. Radiologic investigation may demonstrate evidence of bone destruction and symphysis separation. The etiology of this condition is unclear, but most cases are noninfectious. Suggested therapy includes rest, physical therapy, and nonsteroidal anti-inflammatory agents and, if necessary, steroids. The clinical course may be prolonged but is typically self-limiting. When conservative therapy fails to result in symptom relief, pubic osteomyelitis should be considered and a biopsy with bacterial culture performed. Kammerer-Doak et al found positive cultures in 71% of patients with clinical osteitis pubis who failed to respond to conservative therapy (69). Pubic osteomyelitis is treated with antibiotics and if necessary débridement and/or symphyseal wedge resection.
TRADITIONAL SLING PROCEDURES
Traditional sling procedures, sometimes referred to as pubovaginal slings or bladder neck slings, have undergone a considerable number of modifications since their earliest description in beginning of the 20th century. The fundamentals of the procedure have changed little, however: a strap of material, whether biologic or synthetic, is placed suburethrally at the level of the bladder neck, and the arms are passed behind the symphysis pubis and fixed to the rectus fascia or pubic bone using a combined abdominal-vaginal approach. The newer minimally invasive midurethral slings such as the TVT represent a significant evolution from the traditional sling procedures and are described later in the chapter. As noted previously, traditional sling procedures were classically reserved for use as salvage operations in patients who had failed previous continence surgery or for patients with significant sphincter deficiency. More recently, traditional sling procedures have been advocated for the primary treatment of SUI with urethral hypermobility. A survey of practice patterns in 2000 found that sling procedures were the most common SUI surgery performed by urologists in the United States (70). In 1997, the American Urological Association (AUA)-sponsored Female Stress Urinary Incontinence Clinical Guidelines Panel evaluated the published outcomes data on surgical procedures to treat female SUI (71). The panel concluded, based on the available evidence at the time, that sling procedures, along with retropubic colposuspensions, are the most efficacious procedures for long-term success (71). More recently, the Third International Consultation on Incontinence concluded that “autologous slings provide effective long-term cure for stress incontinence” (24). They were more hesitant in their conclusions regarding slings that use allograft or xenografts, however, because they “have yet to show long-term cure rates equivalent to those reported for autologous fascia” (24).
Mechanism
Although there is some debate regarding the mechanism of action of traditional sling procedures, they are generally thought to restore continence through two mechanisms: (a) re-establishing UVJ position and support and (b) providing a stable suburethral base that results in mechanical compression of the proximal urethra during stress. In patients with SUI and urethral hypermobility, bladder neck slings reposition the UVJ into its normal position and prevent proximal urethral descent during stress (72). As with retropubic colposuspensions, this results in increased pressure transmission to the urethra, relative to the bladder, during elevations in intra-abdominal pressure, thereby
promoting continence (73,74). Consistent with this, Summit et al noted that the success rate of traditional sling procedures is compromised in patients without urethral hypermobility (75). Unlike retropubic operations, sling procedures also create a hammock underneath the proximal urethra that allows mechanical compression of the urethra during stress. When the tightness or anterior elevation of a sling is increased, it results in increased mechanical compression of the urethra and greater urethral resistance. While this promotes continence, it is also can result in voiding dysfunction, the most common complication of sling procedures. Because of this, it is generally recommended that slings be placed loosely so that there is no tension on the urethra at rest. In rare instances where urethral function is completely compromised, resulting in continuous incontinence at rest, it may be desirable to provide greater urethral compression or even complete obstruction as long as the patient is willing to permanently selfcatheterize.
promoting continence (73,74). Consistent with this, Summit et al noted that the success rate of traditional sling procedures is compromised in patients without urethral hypermobility (75). Unlike retropubic operations, sling procedures also create a hammock underneath the proximal urethra that allows mechanical compression of the urethra during stress. When the tightness or anterior elevation of a sling is increased, it results in increased mechanical compression of the urethra and greater urethral resistance. While this promotes continence, it is also can result in voiding dysfunction, the most common complication of sling procedures. Because of this, it is generally recommended that slings be placed loosely so that there is no tension on the urethra at rest. In rare instances where urethral function is completely compromised, resulting in continuous incontinence at rest, it may be desirable to provide greater urethral compression or even complete obstruction as long as the patient is willing to permanently selfcatheterize.
The importance of the suburethral portion of the sling for promoting continence was recently questioned in an interesting animal experiment. Using a rat sling model, Hijaz et al performed slings on 40 animals with SUI (76). Half of the rats received intact slings and the other half received slings in which the suburethral portion was cut at the time of the initial operation. Six weeks after surgery, there was a significant improvement in leak point pressures in both groups compared to animals who received sham operations, and there was no difference between the intact or cut sling groups (76). This implies that, in rats, the lateral arms of the sling are more important for restoring urethral function than the suburethral portion of the sling. Whether these findings translate to humans is currently unknown.
Technique
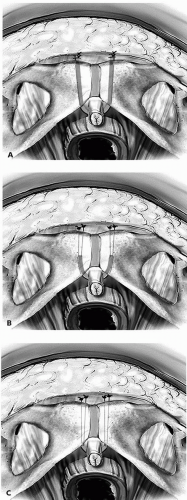
Although a myriad of different sling techniques have been described, they all follow the same fundamental principles, with the critical variables being: (a) the length of the sling (full-length versus a smaller sling or “patch” that is placed suburethrally and fixed via suspending sutures); (b) the type of sling material (autograft, allograft, xenografts, or synthetic); and (c) the point of fixation (rectus fascia, pubic bone, or Cooper’s ligament). Additionally, some surgeons feel that it is important that the sling arms penetrate the periurethral tissues to enter the retropubic space, while others feel this is not necessary (Fig. 14.3). To date, there have been no randomized trials comparing the various sling alternatives, and with few exceptions the existing evidence does not favor one technique over another. As such, the sling technique and choice of material can be left to the discretion of the individual surgeon. A discussion of the advantages and disadvantages of the different sling materials can be found later in the chapter.
The most common autologous tissues used for traditional sling procedures are fascia lata and rectus fascia. If a surgeon chooses to perform an autologous sling, the tissue is typically harvested at the beginning of the procedure, prior to any vaginal dissection. To harvest fascia lata, the patient is placed in the lateral decubitus position with the hip and knee flexed, each at approximately 45 degrees. The leg is prepped from above the hip to below the knee. A 3- to 4-cm incision is made either horizontally or vertically just above the lateral femoral condyle. Dissection is carried down to the underlying fascia lata and the fat is cleaned off with blunt dissection. For a full-length sling, a fascial stripper is used. Typically a 2-cm × 20- to 25-cm strip of fascia lata can be obtained. For a patch sling, the desired graft size, usually 2 cm × 6 to 8 cm, can easily be harvested through the small leg incision. There is no need to reapproximate the fascial defect. After obtaining hemostasis, the subcutaneous tissues are reapproximated, the skin is closed, and a pressure dressing is applied. If a full-length sling has been harvested, it is prudent to apply a pressure dressing to the entire thigh to prevent hematoma formation. To harvest rectus fascia, a low transverse abdominal incision is made two fingerbreadths above the pubic symphysis. Using blunt dissection the fat is dissected off underlying rectus fascia to provide exposure to the harvest site. A strip of rectus fascia of the desired size is harvested in a transverse direction using sharp dissection. Published reports describe harvesting grafts ranging in size from full-length strips of 20 cm in length (77) to patch slings as small as 4 cm in length (78). The typical width is 1 to 3 cm. The fascial incision is closed with No. 0 delayed absorbable suture and the abdominal incision is packed until the vaginal portion of the procedure is completed.
After a decision is made about the type of sling material to be used and, in the case of autologous graft, the material has been harvested, the patient is placed in dorsal lithotomy position in high stirrups and the vagina and lower abdomen are prepped and draped. A Foley catheter is inserted and placed to dependent drainage. Preoperative antibiotics should be administered on-call to the operating room and antithrombotic compression devices applied. If rectus fascia has not been previously harvested, a 4-cm low transverse abdominal incision is made just above the pubic bone, carried down to the rectus fascia, and then packed. Attention is turned to the vagina, where a midline incision or an inverted-U incision is made in the vaginal epithelium from the distal urethra to just beyond the UVJ. The vaginal epithelium is dissected from the underlying tissues laterally to the inferior lateral aspect of the pubic rami. Transvaginal perforation of the endopelvic fascia along the posterior surface of the inferior pubic ramus to enter the retropubic space is accomplished using blunt or sharp dissection on each side of the urethra. Once accomplished, the surgeon should be able to easily pass a finger along the back side of the pubic bone to the inferior aspect of the rectus muscle. During this dissection, care should be taken to remain lateral to the urethra and medial to the pubic tubercle to avoid injury to adjacent structures.
Two small stab incisions are made in the rectus fascia just above the pubic symphysis on either side of the midline. Uterine dressing forceps or a needle ligature carrier is passed through the stab incision, behind the pubic bone, and into the vaginal field on each side of the urethra under the guidance of the surgeon’s vaginal finger. For a full-length sling, the arms of the sling are grasped by the forceps or attached to the ligature carrier and pulled into the abdominal field. For a patch sling, permanent sutures are fixed to each end of the graft and it is the sutures that are grasped and pulled through to the abdominal field. These permanent sutures will act as a “suture bridge” between the sling and the fixation point at the rectus fascia. The midportion of the sling is placed under the proximal urethra at the level of the UVJ. Some surgeons secure the sling in this location with two to four sutures, while others leave the suburethral portion of the sling unattached. The sling is placed at the desired tension and the arms or suture bridges are secured to the rectus fascia. Cystoscopy is performed to ensure that no bladder or urethral injury has occurred. The abdominal and vaginal incisions are closed. The bladder is drained transurethrally or suprapubically until normal voiding resumes.
Some surgeons prefer to fix the sling arms to the pubic bone or Cooper’s ligament rather than the rectus fascia. This can be accomplished using bone anchoring devices or, for fixation to Cooper’s ligament, a curved Capio needle driver (Boston Scientific, Natick, MA) The use of these alternative fixation points allows the sling to be placed entirely through the vaginal incision while avoiding the need for the abdominal incision. The potential advantages and disadvantages of using bone anchors have been reviewed by several authors (79, 80, 81).
Perhaps the most important step when performing a traditional sling procedure is determining how much tension to apply to the sling when securing the sling arms. Numerous techniques have been described to determine optimal sling tension, including measuring the degree of urethral deflection with a Q-tip, placing a spacer such as a rightangle clamp, Hegar dilator, or cystoscope sheath between the sling and the urethra, and even intraoperative urodynamic assessment of urethral function (82, 83, 84, 85). Unfortunately, there is no standardized technique that can be applied to all patients. Generally, slings should be placed loosely so that there is no compression of the urethra at rest. As mentioned previously, in a patient with severe incontinence and compromise to urethral function, it may be desirable to tension the sling tighter so that it is purposefully obstructive. However, these instances are rare and the patient must be willing to accept the possibility of voiding dysfunction requiring regular self-catheterization.
Outcomes
Although one of the most popular procedures for the treatment for SUI, the quality of the evidence regarding the efficacy of traditional sling procedures is, somewhat surprisingly, considerably less than that of retropubic colposuspensions. The Cochrane Collaboration review of traditional sling procedures in 2005 identified 13 randomized trials involving a total of 760 women evaluating this procedure (55). Most of the studies were of small size (n = 20 to 165) and poor quality (55). That being said, the level 1 evidence that does exist, along with a large volume of retrospective and prospective cohort studies, do support the conclusion that traditional sling procedures are effective in the management of SUI. Five randomized trials have compared the traditional sling to the retropubic colposuspension, and in each there was no difference in efficacy between the two procedures (86, 87, 88, 89, 90). In the majority of the nonrandomized studies in the literature, traditional sling procedures were used as salvage surgery in women who had failed previous continence surgery. In this capacity, the objective cure rates reported in the literature range from 61% to 100%, with a mean cure rate of 85% (91%). While there are fewer studies evaluating traditional slings as first-line therapy for SUI, the reported cure rates as a first operation are 87% to 94% (24,91). As mentioned, the UITN’s SISTEr trial, which has randomized 650 women with SUI to either rectus fascia sling or Burch colposuspension, should provide valuable information about the relative merits of these two procedures (56).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree