Lower Urinary Tract Infection
Mickey M. Karram
Sam Siddighi
Urinary tract infections (UTIs) in women produce significant health problems. They are among the most common infections dealt with by primary care physicians. Although rarely followed by severe sequelae, sometimes they lead to acute pyelonephritis and bacteremia and become a major cause of morbidity and time lost from work.
The proper management of these patients, although often simple, has recently been challenged by several occurrences: (a) the introduction of new antimicrobial agents, (b) the advent of single-dose therapy, (c) the recognition of additional lower urinary tract pathogens such as Staphylococcus saprophyticus and Chlamydia trachomatis, (d) the realization that many women with symptomatic cystitis may have less than 105 organisms/mL in urine cultures; and (e) the understanding that certain patients with infection-like symptoms will be considered to have urethral syndrome, painful bladder, or even interstitial cystitis because they have no apparent cause for their symptoms.
PREVALENCE
About 5 million cases of acute cystitis occur annually in the United States, resulting in an estimated 6 million office visits (1). The overall expenditure for the treatment of UTIs in women in the United States, excluding outpatient medication prescriptions, was approximately $2.47 billion in the year 2000 (2).
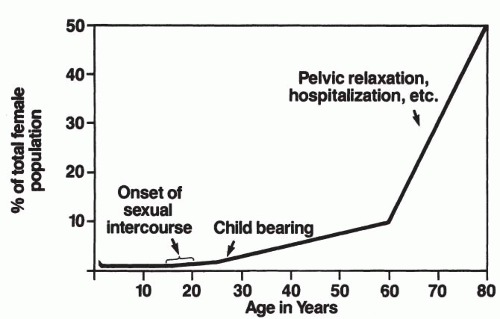
UTIs are much more prevalent among women than men (ratio of 8:1). This is probably secondary to an anatomically short urethra in proximity to a large bacterial reservoir within the introital tract and along the vaginal vestibule (3). The incidence of UTIs rises with age. At 1 year of age, there is an approximately 1% to 2% incidence of bacteriuria in females; pathology directly correlates with these infections. As many as 50% of patients show abnormalities on intravenous pyelogram (IVP) that is scarring and either ipsilateral reflux or some obstructive disease (4,5). After 1 year of age, the infection rate decreases to about 1% and continues to decrease until puberty. The incidence of urologic pathology associated with these infections also continues to decrease progressively. With the introduction of sexual activity and pregnancy, the incidence starts to rise and continues to increase progressively with age. Between the ages of 15 and 24 years, the prevalence of bacteriuria is about 2% to 3% and increases to about 10% at the age of 60 years, 20% after the age of 65 years, and 25% to 50% after the age of 80 years (6) (Fig. 10.1). Additionally, more than 50% of menopausal women will experience some symptoms of urogenital atrophy and UTI (7).
About 2% of all patients admitted to a hospital acquire a UTI during their stay, which accounts for 500,000 hospital-acquired UTIs per year. One percent (5,000) of these infections become life-threatening. Instrumentation or catheterization of the urinary tract is a precipitating factor in at least 80% of these nosocomial infections (8,9).
DEFINITIONS
Before discussing UTI, an understanding of generally accepted definitions is essential because the commonly used terminology can, at times, be confusing.
Cystitis indicates inflammation of the bladder, whether used as a histologic, bacteriologic, cystoscopic, or clinical description. Most commonly, it produces symptoms of urinary frequency and dysuria. Bacterial cystitis needs to be differentiated from nonbacterial cystitis (e.g., radiation, interstitial).
Urethritis refers to inflammation of the urethra and usually requires an adjective for modification (e.g., chlamydial, nonspecific). In female patients, symptoms of urethritis are impossible to distinguish from those of cystitis.
Trigonitis is inflammation or localized hyperemia of the trigone. This term is commonly used to describe the normal cobblestone or granular appearance of the trigone and floor of the vesical neck. The failure to recognize that this epithelium is part of the normal embryologic development and the lack of experience in cystoscopic examinations of normal women without bladder symptoms are probably responsible for the terms “trigonitis” and “granular urethral trigonitis.”
Bacteriuria implies the presence of bacteria in the bladder urine and not contaminants that have been added to sterile bladder urine. The term includes both renal and bladder bacteria. Lower UTI can be defined as bacteriuria of greater than 102 colony-forming units per milliliter (cfu/mL) in the presence of symptoms, or asymptomatic bacteriuria with the growth of 105 cfu/mL or more.
Urethral syndrome is a poorly defined syndrome of frequency, urgency, dysuria, suprapubic discomfort, and voiding difficulties in the absence of any organic pathology. This term needs clarification, and it should not be used to describe urine with bacterial counts of less than 105 organisms/mL, chlamydial infection of the urethra, or a hypoestrogenic urethra. When we use the term “urethral syndrome,” we have ruled out detrusor and urethral dysfunction as well as any lower UTI. Thus, it is basically a “wastebasket” diagnosis of lower urinary tract symptoms without any discernible pathology (10,11).
PATHOGENESIS
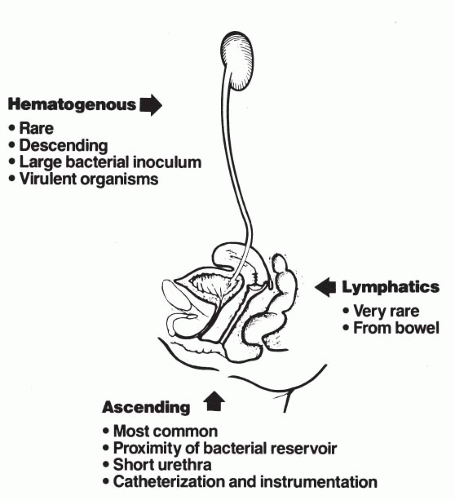
The pathogenesis of UTI in female patients has been postulated to involve three primary mechanisms: hematogenous, lymphatic spread, or ascending extension of organisms directly from the rectum (Fig. 10.2). Retrograde (ascending) infection is the most widely accepted mechanism and appears to be important in the management of infections. Hematogenous dissemination is the principal route by which staphylococcal organisms seed the kidney. This leads to pyelonephritis and may be an important route for patients who do not have vesicoureteral reflux.
The normal female urinary tract is remarkably resistant to infection. Although certain risk factors for developing UTIs have been identified (Table 10.1), it remains unclear why certain women are more prone to infection. Individual differences at the molecular level (i.e., genetic differences and production of inhibitory substances) may account
for the aforementioned (12). Susceptibility probably also depends on the inoculum size, the virulence properties of the invading microorganism, and, most importantly, the status of the defense mechanisms of the host. These host mechanisms are found in the urine, the vagina, and throughout the female urinary tract.
for the aforementioned (12). Susceptibility probably also depends on the inoculum size, the virulence properties of the invading microorganism, and, most importantly, the status of the defense mechanisms of the host. These host mechanisms are found in the urine, the vagina, and throughout the female urinary tract.
TABLE 10.1 Known Risk Factors for UTI | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The Enterobacteriaceae are responsible for about 80% of bacteriuria in UTIs. Escherichia coli accounts for the majority of the community-acquired infections; other organisms are responsible for a disproportionate number of infections, considering their frequency in stool flora. Klebsiella species cause about 12% of infections, whereas Enterobacter and Proteus species together account for another 12% of infections outside the hospital. Serratia marcescens and Pseudomonas aeruginosa are almost always hospital-acquired and are due to omission of infection control practices, usually after urethral catheterization or manipulation. Although anaerobes are present in abundance in the feces of normal individuals, they are rarely the cause of UTI. The oxygen tension in the urine probably prevents their growth and persistence within the urinary tract. Staphylococcus epidermidis is also a cause of nosocomial UTI in catheterized patients and is frequently resistant to antibacterial agents (13, 14, 15, 16, 17, 18). Other gram-positive organisms, including the group B (Staphylococcus agalactiae) and group D streptococci (Enterococcus), cause 1% to 2% of UTIs.
In summary, based on the most recent North American Urinary Tract Infection Collaborative Alliance (NAUTICA) study results, prevalence rates based on outpatient urinary isolates from 41 medical centers are as follows: E. coli (57.5%), Klebsiella pneumoniae (12.4%), Enterococcus spp. (6.6%), Proteus mirabilis (5.4%), P. aeruginosa (2.9%), Citrobacter spp. (2.7%), Staphylococcus aureus (2.2%), Enterobacter cloacae (1.9%), coag-ulase-negative staphylococci (1.3%), S. saprophyticus (1.2%), other Klebsiella spp. (1.2%), Enterobacter aerogenes (1.1%), and Streptococcus agalactiae (1.0%) (19).
Bacteria are not the only organisms that can infect the lower urinary tract. Yeast can be identified in the urine culture of some hospitalized patients at a concentration of above 103 yeast colonies/mL. The most common predisposing factors are antibiotic therapy and an indwelling catheter, but diabetes mellitus and immunocompromised conditions are also strong risk factors. Candida albicans is the predominant organism responsible for candiduria in susceptible patients. Other Candida spp. as well as Torulopsis glabrata may lead to UTI. Rarely, trematodes such as Schistosoma haematobium and tapeworms such as Echinococcus granulosus (hydatid cysts) may also infect the lower urinary tract, especially the bladder (20).
HOST DEFENSE MECHANISMS
Urine
Urine has certain defense mechanisms against infection. The most important inhibitory factors include a very high osmolality (i.e., high urea concentration) and a high organic acid concentration (i.e., low pH). Both of these reduce bacterial growth by inhibiting phagocytosis and decreasing the reactivity of complement. In general, anaerobic bacteria and other fastidious organisms that make up most of the urethral flora do not multiply in urine. However, urine usually supports growth of nonfastidious bacteria (21,22).
Vaginal, Periurethral, and Perineal Colonization
There is accumulating evidence that the antibacterial defense mechanisms of the vaginal walls and periurethral area are important in preventing the progression of microorganisms from the rectum to the bladder. Normally, this area is colonized by gram-positive bacteria, lactobacillus, and diphtheroids
(organisms that grow very poorly in urine and do not cause UTIs). A number of studies have shown that females with recurrent cystitis first colonize their vaginal introitus and periurethral area with enterobacteria before the onset of the symptoms of cystitis and then are at risk for infection until this colonization reverses to a normal situation (4,21,23). Acidity of vaginal secretions may contribute to vaginal resistance to coliform bacteria. In premenopausal females, the vaginal pH is usually near 4.0. This low acidic pH prohibits the growth of organisms such as E. coli but promotes the growth of the normally present organisms (e.g., lactobacillus) that will interfere with the growth of uropathogens (24,25). High vaginal pH appears to be associated with the growth of enterobacteria (26). Treatment of menopausal patients with intravaginal estrogen leads to reappearance of lactobacilli, decline in vaginal pH, decrease in growth of uropathogens, and reduction in the incidence of UTI (27,28).
(organisms that grow very poorly in urine and do not cause UTIs). A number of studies have shown that females with recurrent cystitis first colonize their vaginal introitus and periurethral area with enterobacteria before the onset of the symptoms of cystitis and then are at risk for infection until this colonization reverses to a normal situation (4,21,23). Acidity of vaginal secretions may contribute to vaginal resistance to coliform bacteria. In premenopausal females, the vaginal pH is usually near 4.0. This low acidic pH prohibits the growth of organisms such as E. coli but promotes the growth of the normally present organisms (e.g., lactobacillus) that will interfere with the growth of uropathogens (24,25). High vaginal pH appears to be associated with the growth of enterobacteria (26). Treatment of menopausal patients with intravaginal estrogen leads to reappearance of lactobacilli, decline in vaginal pH, decrease in growth of uropathogens, and reduction in the incidence of UTI (27,28).
Normal Periodic Voiding
Periodic voiding is one of the most important known bladder defense mechanisms. One study noted the introduction of 10 million bacteria into normal male bladders failed to establish infection because the organisms were rapidly cleared by voiding, diluting with fresh urine, and voiding again (29). Voiding displaces infected urine with sterile urine and flushes out bacteria attached to desquamated uroepithelial cells. In addition, a thin film of urine remains in the bladder after emptying and any bacteria present are removed by the mucosal cell production of organic acids.
Unfortunately, episodic voiding is not enough to prevent infection after sexual intercourse. In case-controlled studies, voiding patterns before and after sexual activity were not associated with recurrent UTIs (30).
Prevention of Bacterial Adherence
The ability of an organism to bind to the epithelial cell has been shown to correlate with its ability to infect the urinary tract. The ascending loop of Henle secretes Tamm-Horsfall protein, which is a uromucoid, rich in mannose. This protein may inhibit bacterial adherence and trap bacteria in the urine, allowing them to be flushed from the urinary tract (31). Also, the presence of urinary immunoglobulin and the lining of the bladder with a glycosaminoglycan may be important factors in the blocking of bacterial adherence. The reduction of glycosaminoglycan probably plays a role in recurrent cystitis (32,33).
HOST SUSCEPTIBILITY FACTORS
Bacterial Adherence
Adherence of microorganisms to mucosal cells is considered to be a prerequisite for colonization and infection (34). As previously mentioned, when these organisms enter the urethra and bladder in most women, they do not adhere and are easily washed away. In patients who are susceptible to UTIs, the organisms will quickly lock into the defective epithelial cells. The fecal flora is almost invariably the source of the infecting organisms. E. coli is the major pathogen, although S. epidermidis and Enterococcus, Klebsiella, and Proteus species can sometimes be identified (Fig. 10.3). The interaction of the mucosal and bacterial cells is probably dependent on both receptors on the mucosa and some type of attachment mechanism used by the bacteria. E. coli has been shown to possess surface organelles that mediate attachment to specific host receptors. These structures are called pili and can be present in large numbers on the microbial cell. Two types that appear to be important in urinary infections have been identified. Type I pili seek mannose as a receptor and are isolated from individuals with cystitis. They tend to bind with a low affinity, and their presence is not correlated highly with pathogenicity. Type II pili are man-nose-negative or “p pili” and adhere to the P blood group antigens. E. coli strains possessing p fimbriae are more virulent and more likely to cause pyelonephritis than strains without them (35, 36, 37).
Schaeffer et al (38) studied the adherence of E. coli to vaginal epithelial cells in control subjects and in women who had experienced at least three UTIs in the past year. They found adherence to be
greater in the study patients than in the controls. The vaginal cells of those receiving a sustained course of antimicrobial showed less adherence than the vaginal cells of patients who were not taking antibiotics. If the antibiotics were discontinued, adherence returned, and reinfection usually occurred (39). In another study, Schaeffer et al (40) noted that adherence tended to be higher during the early estrogen-dependent phase of the menstrual cycle.
greater in the study patients than in the controls. The vaginal cells of those receiving a sustained course of antimicrobial showed less adherence than the vaginal cells of patients who were not taking antibiotics. If the antibiotics were discontinued, adherence returned, and reinfection usually occurred (39). In another study, Schaeffer et al (40) noted that adherence tended to be higher during the early estrogen-dependent phase of the menstrual cycle.
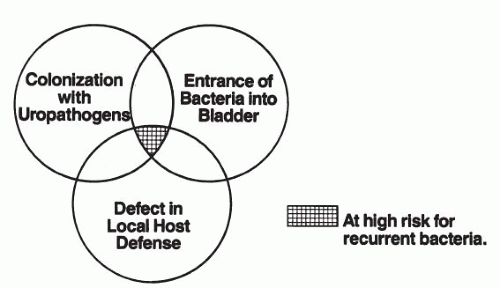 FIGURE 10.3 ● Factors determining host risk and susceptibility to bacterial cystitis in normal females with anatomically normal urinary tracts. |
Furthermore, women at high risk for recurrent UTIs may be more genetically prone to recurrent infection. Although the mechanism is not understood, women who bear human leukocyte antigen A3 subtype (HLA-A3) are more likely to have had recurrent UTIs than those who lack this antigen (41). Other work also suggests that women of blood group B or AB who are nonsecretors of blood group substances are at significantly higher risk for developing infections than are women of other blood groups (42). In addition, patients with Lewis blood group types who are considered secretors have a lower incidence of UTIs. The Lewis blood groups exists at two genetic loci: Lea and Leb. Secretors possess the Lewis “b” genetic locus (i.e., Le(a+,b+) and Le(ā,b+), whereas nonsecretors are 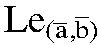 ). Evidence suggests that bacteria are unable to adhere to the urothelial cell because of alterations to the uromucoid, which inhibits binding (42, 43, 44, 45, 46).
). Evidence suggests that bacteria are unable to adhere to the urothelial cell because of alterations to the uromucoid, which inhibits binding (42, 43, 44, 45, 46).
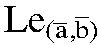 ). Evidence suggests that bacteria are unable to adhere to the urothelial cell because of alterations to the uromucoid, which inhibits binding (42, 43, 44, 45, 46).
). Evidence suggests that bacteria are unable to adhere to the urothelial cell because of alterations to the uromucoid, which inhibits binding (42, 43, 44, 45, 46).Thus, these genetic differences at the cellular level appear to influence bacterial adherence and make certain women more prone to UTIs.
Sexual Intercourse
In women, sexual intercourse appears to be a major determinant for bacterial entry into the bladder. Prospective studies have shown that many UTIs develop the day after sexual intercourse (47). Both the frequency and recency of sexual intercourse increase the risk for UTI. It has been shown that women who have engaged in sexual intercourse within the prior 48 hours have a risk for infection 60 times greater than women who have not (47). Sexual intercourse with a new partner within the past year is also another independent risk factor.
Infection appears to occur through inoculation of periurethral bacteria into the bladder during active intercourse. Women who have not colonized their vaginal and periurethral areas with coliform bacteria will have introduction of normal vaginal flora (e.g., lactobacillus, diphtheroid, or S. epidermidis), which will not produce infection and are rapidly cleared with voiding. However, in the colonized women, the pathogenic organisms, such as E. coli, will infect the bladder. There is evidence that women who have recurrent UTI have shorter distances from the urethral meatus and posterior fourchette to the anus (48). Another commonly overlooked factor is the use of diaphragms. A number of studies have confirmed that diaphragm users are at increased risk for UTI even after statistically controlling for sexual activity and history of previous UTI (49,50). The mechanism is unknown; it is believed that it may be related to urethral obstruction caused by the diaphragm (51,52). Also, diaphragm users have reduced vaginal colonization with lactobacillus, but coliforms are isolated three times more often than in women using other contraceptive methods (50). Additionally, the spermicidal agent nonoxynol-9 is an independent risk factor for UTIs. Spermicides reduce vaginal lactobacilli, allowing growth of uropathogens.
Systemic Factors
Diabetic patients are prone to develop neurogenic bladder dysfunction and severe vascular disease, both of which can predispose to UTIs. Other genetic problems that are commonly associated with UTIs are gouty nephropathy, sickle cell trait, and cystic renal disease.
It must be understood that the explanations mentioned for the pathogenesis of UTIs apply only to those females who have normal urinary tracts. Bacteria in the presence of obstructions, stones, or a neurogenic bladder do not need to have special invasive properties other than the ability to grow in urine.
CLINICAL PRESENTATION
The signs and symptoms of UTI in females can be diverse. It is helpful to distinguish lower UTI (cystitis) from upper tract infection (pyelonephritis) to aid in the selection of proper antimicrobial therapy and to plan appropriate follow-up.
The most common symptom of uncomplicated UTI is frequency of urination, present in 94% of patients (53). Cystitis is also manifested by lower urinary tract irritative symptoms such as dysuria, urgency, nocturia, suprapubic discomfort, low backache, and even flank pain. Urinary incontinence occasionally may be a symptom of UTI. This may be due to the urethral sphincter relaxation mediated by E. coli endotoxin (54). Uncommonly, one can have gross hematuria. Systemic symptoms such as fever and chills are usually absent in lower UTIs. One should be aware that the elderly may have more subtle symptoms
such as malaise, mild abdominal pain, nocturia, and urinary incontinence instead of the classic symptoms discussed above.
such as malaise, mild abdominal pain, nocturia, and urinary incontinence instead of the classic symptoms discussed above.
Upper tract infections involving the renal pelvis, calyces, and parenchyma commonly present with fever, chills, malaise, and occasionally (especially in elderly patients) nausea and vomiting. Costovertebral angle tenderness and flank pain are usually present. However, it should be noted that because of referred pain pathways, lower UTIs may also be accompanied by flank pain and costovertebral angle tenderness. There is colicky pain if acute pyelonephritis is complicated by either a renal calculus or a sloughed renal papilla secondary to diabetic or analgesic nephropathy. More detailed discussion of upper UTI is beyond the scope of this chapter.
DIAGNOSIS OF BACTERIURIA
Before performing tests to document the presence or absence of pathogenic bacteria in the urine, the method of urinary collection must be considered. Considerable care must be taken in the collection of urine from ambulatory females. Kass (55,56) published results demonstrating that one whole voided urine specimen with a colony count of greater than 105 cfu/mL has only an 80% chance of representing true infection. Three specimens increased the odds to 95% (55,56). Even when intelligent, educated patients are given clear, detailed instructions for collection of urine, errors can occur. Certain patients, because of physical disability or obesity, are simply unable to obtain a clean voided specimen without assistance. When necessary to avoid these limitations, specimens can be obtained by urethral catheterization. Additionally, the patient can lie in the lithotomy position on an examining table and void after the perineum is cleaned with soap and water while the nurse collects a midstream specimen. Sometimes, bladder urine may need to be aspirated suprapubically (57). Although urethral catheterization is the most time-honored method, it should be kept in mind that catheterization is not without risks. Reports have noted that catheter-induced infection rates range from 1% in young, healthy females to as high as 20% in hospitalized females (58,59).
Urine Microscopy
Microscopic analysis of urine is an easy and valuable method of evaluating women with symptoms of UTI. A thorough microscopic examination of an uncentrifuged sample of urine showing minimal epithelial cells (thus not contaminated), bacteria moving in the field, and 2 to 6 leukocytes per highpower field correlates with above 106 cfu/mL on culture. If infection with greater than 104 cfu/mL is present, the finding of one or more bacteria on a Gram-stained specimen of urine correlates highly with the presence of UTI, having a sensitivity of 80% and a specificity of 90% with a positive predictive value of about 85% (60). Gram stain of the urine is useful in detecting abundant bacteriuria but is of little help in infection with colony counts of less than 104 cfu/mL.
Fresh, unspun urine should also be quantitatively assessed with a hemocytometer for the number of white blood cells. The hemocytometer is positioned on the microscope stage. The number of leukocytes is counted in each of nine large squares, divided by 9 and multiplied by 10 to yield the number of white blood cells per milliliter. Pyuria is defined as greater than 10 leukocytes/mL. Pyuria is present in nearly all women with acute UTI. Studies note the presence of pyuria to be 80% to 95% sensitive (even when bacteria counts are less than 104) and 50% to 75% specific for the presence of UTI. However, a study of pregnant patients presenting acutely to a labor ward showed that only 17% of patients with significant pyuria had a significant urine culture (61). It is also of value to ascertain whether red blood cells are present or to perform a urine dipstick for blood. Microscopic hematuria can be found in about 50% of women with acute UTI and is rarely present in patients who have dysuria from other causes (62,63).
Office Urine Kits
If expertise for office microscopy is not available or feasible, it is reasonable to substitute a rapid diagnostic test for bacteriuria, pyuria, and hematuria, although in general these lead to less accurate results than microscopy.
The most common rapid detection test is the nitrite test. Certain bacteria such as Proteus species and occasionally E. coli have the enzyme nitrate reductase, which converts dietary nitrates into nitrite; this, in turn, causes the amine-impregnated dipstick to turn pink within 60 seconds of reaction. Numerous commercial urine dip tests are available, and one should check the sensitivity and specificity of the particular test kit used. Generally, a positive nitrite test is highly specific (92% to 100%) for UTI and deserves treatment. However, the test is not sensitive and is not a good screening tool (i.e., only 25% of patients with UTI test positive for nitrites). Lack of dietary nitrate, organisms that lack nitrate reductase, and diuretics can lead to false-negative results.
The nitrite test is often integrated with a test for leukocyte esterase (LE), which is an enzyme found in primary neutrophil granules. When LE reacts with reagents impregnated in the dipstick, a blue color is produced within 1 to 2 minutes, indicating a positive test. The LE test has a specificity of 94% to 98% (64). However, the sensitivity of the LE test is directly related to the bacterial load. Wu et al (65) showed a sensitivity of only 22% in infections with 104 to 105 cfu/mL versus 60% for those with greater than 105 cfu/mL. In other words, low-level pyuria (5 to 20 white blood cells per highpower field microscopy) may be associated with a false-negative LE test. These tests are also best performed on concentrated first-morning voided specimens. It has been suggested that false-negative results are more likely if the test is used as a sampling technique at other times during the day (66). Furthermore, certain dyes such as bilirubin, methylene blue, or phenazopyridine may interfere with interpretation of the test.
Other rapid detection tests, such as filter methods (e.g., Back-T-Screen, Marion Laboratories, Inc., Kansas City, MO), concentrate a specific quantity of urinary sediment on a filter of controlled pore size. One milliliter of urine is mixed with 3 mL of a diluent containing glacial acetic acid and other ingredients that dissolve crystals and increase adherence of bacteria and leukocytes. The diluted mixture is then passed through the filter and rinsed with a diluent. A safranin dye is then used to stain the bacteria and leukocytes, and a decolorizer is added to remove excess dye. Resulting colors are compared with a reference to quantitate the presence of bacteria and leukocytes. The sensitivity of these tests for urine infected with 104 to 105 cfu/mL is 34% to 65%. As the number of organisms increases to greater than 105, the sensitivity also increases to 79% to 85%. The specificity of this test at lower bacterial counts is about 75% (65,67). The main advantage of these tests is a more reliable detection of smaller numbers of bacteria at the expense of lower specificity (68). The test is believed by some to be a good screening method because it detects both bacteria and pyuria.
A symptomatic patient should be treated with antibiotics even if an office urinary kit is negative for both nitrites and leukocytes. Empiric antibiotic use is guided by symptoms because treatment leads to faster resolution of lower urinary tract symptoms and will reduce the median duration of constitutional symptoms (fever and shivers) by 4 days (69). Additionally, treatment can reduce time of restricted activity and leave from work.
Urine Culture
In the patient who has clinical signs of acute lower UTI and is noted to have pyuria, bacteriuria, or hematuria on one of the previously mentioned office tests, it is reasonable to initiate antibiotic therapy without obtaining a urine culture. However, if one of the screening techniques is deemed inappropriate or inconclusive, if the patient has recurrent infection that has not been subjectively relieved with previous antibiotics, or if signs and symptoms are consistent with upper UTI, a bacterial culture and sensitivity should be performed.
The traditional approach to the interpretation of a urinary culture has been that there must be growth of at least 105 cfu/mL to consider it positive. This criterion is based on studies demonstrating that the finding of at least 105 cfu/mL on two consecutive urine cultures distinguishes women with asymptomatic bacteriuria or pyelonephritis from those with contaminated specimens (55,56,60).
The use of this cutoff, however, has two limitations for the clinician who treats these patients. First, 20% to 24% of women with symptomatic urinary infections present with less than 105 bacteria/mL of urine (57,70, 71, 72). This is probably secondary to a slow doubling time of bacteria in urine combined with frequent bladder emptying from persistent irritation. Stamm et al (73) proposed that the best diagnostic criterion for culture detection in young symptomatic women is 102 cfu/mL, not 105 cfu/mL.
The second limitation of the 105 cutoff is one of overdiagnosis. In the original studies by Kass (55,56,74), a single culture of at least 105 cfu/mL had a 20% chance of representing contamination. Because patients who are susceptible to infection often carry large numbers of pathogenic bacteria on the perineum, contamination of an otherwise sterile urine can occur. For this reason, care in the collection of the urine specimen must again be emphasized. Most health care workers do not spend much time and effort to explain adequately how a patient should collect a midstream urine clean-catch specimen. However, one study showed contamination rates to be similar among patients whose urine samples were collected with traditional instructions (midstream urine sample, perineal cleansing, and spreading of the labia) compared with urine samples of patients told to urinate into a clean container without cleansing (75).
Although methods of obtaining cultures in the office are available, most clinicians use commercial
laboratories. One should be familiar with the individual laboratory policy of reporting culture results. Some laboratories report any culture of less than 105 cfu/mL as negative and often report only the predominant organism in mixed cultures.
laboratories. One should be familiar with the individual laboratory policy of reporting culture results. Some laboratories report any culture of less than 105 cfu/mL as negative and often report only the predominant organism in mixed cultures.
Sensitivity testing is also usually obtained using a commercial laboratory, even though office tests have been described. The disadvantages of sensitivity testing include the time involved, which is typically 24 to 48 hours; the absence of control of processing by the referring physician; and the relatively high cost.
Cystoscopy
Cystitis may appear as diffuse inflammation (nonraised, red areas distributed throughout) on cystoscopy. Occasionally, one may note one or multiple small clear cysts throughout the bladder from a resolved UTI. This is termed “cystitis cystica.”
Cystoscopy is not routinely indicated for the evaluation of lower UTI, and routine endoscopic evaluation in females with UTI is a controversial issue. Fowler and Pulaski (76) reported on 74 cystoscopies performed in women with two or more previous infections and noted the only abnormality that altered treatment was the presence of a urethral diverticulum in three cases. Engel et al (77) reviewed 153 women who had undergone cystoscopy for UTI. Although abnormalities were noted in 62% of the cases, 84% of these abnormalities were inflammatory in nature and presumably secondary to prior infection. Only one abnormality, a colovesical fistula, had an effect on treatment (77).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree