Patients with constipation and fecal incontinence usually come to the attention of the surgeon when conservative measures have failed to alleviate sufficiently severe symptoms. Following detailed clinical and physiologic assessment, the surgeon should tailor the procedure to specific underlying physiologic abnormalities to restore function. This article describes the rationale, indications (including patient selection), results, and current position controversies of surgical procedures for constipation and fecal incontinence, dividing these into those regarded as historical, contemporary, or evolving. Reported surgical outcome data must be interpreted with caution because for most studies the evidence is of low quality, making comparison of different procedures problematic and emphasizing the need for better designed and conducted clinical trials.
Constipation and fecal incontinence (FI) both represent symptom complexes that in clinical practice present to health care providers when symptoms are sufficiently severe, and to surgeons when first-line, conservative measures have failed. It is important to consider, especially when surgical intervention is being contemplated, that patients with constipation or FI represent heterogeneous populations both in terms of reported symptoms and underlying pathophysiology. Accordingly, detailed assessment of clinical symptoms and their severity and full characterization of underlying physiologic abnormalities are required in individual patients before embarking on potentially irreversible interventions. With such information to hand, the surgeon is then able to tailor the procedure to specific underlying abnormalities to restore normal physiology and it is hoped function. Unfortunately, this is not always possible and, in such cases, treatment is frequently empiric (ie, aimed at reducing symptom severity, rather than restoring normal function).
For the purposes of the following discussion, surgical treatment of constipation and FI are considered separately, although it should be acknowledged, as is being increasingly recognized, that both conditions may coexist in individual patients (eg, outlet obstruction and passive FI). Some procedures may treat FI by improving evacuation. Classically, surgery is considered as a branch of medicine that treats diseases, injuries, and deformities by manual or operative methods. This article includes some therapeutic procedures that are perceived to be less invasive (eg, sacral nerve stimulation [SNS] and injection of biomaterials) but does not include purely diagnostic interventions, such as full-thickness rectal biopsies. For both constipation and FI, the reader is guided by dividing surgical interventions into those regarded as historical, contemporary, or evolving. Provided for each are the (1) rationale; (2) indications (including patient selection); (3) results including complications; and (4) the current position of the procedure in the management armamentarium, especially including controversies. The published success rates, outcome measures, and grades of evidence are summarized for each of these procedures in Tables 1 and 2 . In the most part, the treatment refers to that of adults unless specified. Throughout, it is assumed that organic causes have been excluded.
| Procedure | Success Rate | Outcome Measures | Grade of Evidence |
|---|---|---|---|
| Historical | |||
| Pelvic floor procedures | <50% (17%–48%) | Spontaneous defecation | D |
| Contemporary | |||
| Colectomy | 86% (39%–100%) | Satisfaction ratings, QOLclinical, physiologic | D |
| Anterograde colonic enema | 47% | Satisfaction | D |
| Fecal diversion | No valid data | N/A | N/A |
| Evolving | |||
| Sacral nerve stimulation | 42%–66% | 50% symptom improvement | D |
| STARR procedure | 50%–90% | Overall satisfaction | B/D a |
| Vertical reduction rectoplasty | 70% | Satisfaction, clinical (CCS), physiologic | D |
a One randomized controlled trial available but comparison with another experimental surgical procedure only.
| Procedure | Success Rate | Outcome Measures | Grade of Evidence |
|---|---|---|---|
| Historical | |||
| Pelvic floor procedures | 33%–50% | Full continence | B a |
| Contemporary | |||
| Sphincter repair | 50%–66% b | Clinical, physiologic | B a |
| Sacral nerve stimulation |
| Complete continenceImproved (by 50%) continence | A |
| Dynamic gracilis neosphincter | 42%–85% | Restoration of continence | D c |
| Artificial bowel sphincter | 50%–100% d | Full continence | B a |
| Fecal diversion | No valid data | N/A | N/A |
| Evolving | |||
| Injection of biomaterials | 66% short term e | Cessation leakage and improved continence | D |
| SECCA procedure | 84% | 50% improvement | D |
| Rectal augmentation | 64% | Avoidance of stoma | D |
a Derived from Cochrane review but in some instances data extrapolated from only one study.
b 5-year success rates fall to 50%.
c Systematic review available but only of case series with no comparative studies.
d Explantation rates in case series approximately 50%.
e No change in continence scores compared with preoperatively at long-term follow-up.
Surgical treatment of constipation
Introduction
Patients with constipation usually present to the surgeon when nonsurgical therapies (laxatives, behavioral therapies including biofeedback) have already failed. In practice, this group has a strong female predominance and includes patients who on specialist physiologic investigation commonly have slow colonic transit, severe outlet obstruction, or both. Aside from such interventions as manual evacuation, the mainstay of surgical treatment for slow transit constipation (STC) has been colectomy, whereas outlet obstruction has less clearly defined effective therapies. In respect of the latter, although they may be pathophysiologically associated, the numerous therapies for rectal prolapse and rectocele are not addressed. Table 1 summarizes the procedures that are discussed in detail.
Historical Surgical Treatments
Colectomy, first described for constipation 100 years ago, is still performed and is discussed next. In contrast, a variety of anorectal procedures (eg, anal dilatation, anorectal myectomy, partial division of puborectalis) had been performed with the primary aim of alleviating outlet obstruction in children and adults on the basis that this was caused by sphincter–pelvic floor hypertonia. Despite short-term improvement in up to 60% of patients, long-term results of pelvic floor procedures are disappointing, with success rates of only 48%. In recent years, the concept of paradoxical contraction of the pelvic floor musculature as a cause of constipation has been seriously questioned. Furthermore, there is a risk of incontinence following such interventions.
Contemporary Surgical Treatments
Colonic resection
Resection of all or part of the colon has been described as a treatment for severe constipation since 1908 and for patients with proved slow colonic transit since 1984. Having peaked in popularity in the early 1990s, some more disappointing European long-term results and high complication rates have led to its more cautious application in the twenty-first century. Nevertheless, it continues to be used.
Rationale
The shortened colon reduces colonic transit time and delivers less solid (more easily evacuated) stool to the rectum.
Indications and patient selection
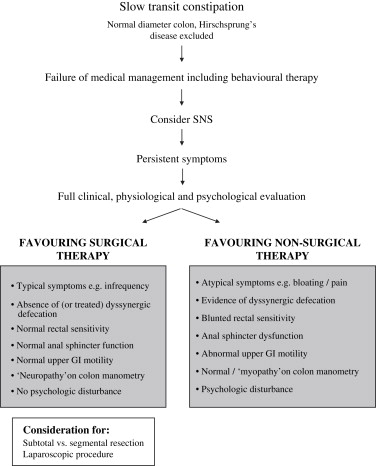
It is now widely accepted that the procedure should be reserved for those with documented slow transit in whom nonsurgical interventions have failed to ameliorate symptoms that are sufficiently severe to affect adversely quality of life. Furthermore, expectations should be clearly defined in relation to outcomes and complications, particularly in respect of the relative lack of efficacy of this procedure in treating abdominal pain and bloating, body image, or psychologic complaints. Fig. 1 demonstrates the factors to be consideration when contemplating surgical intervention. Specific clinical and physiologic findings in relation to patient selection are discussed next.
Results and complications
These were systematically reviewed in 1999. Overall, 32 case series (1981–1998) provided outcome data in 12 to 106 patients. Mortality rates were documented in 23 series, and varied from 0% to 6%. The commonest postoperative morbidity was small bowel obstruction occurring in 2% to 71% patients (median, 18%), and resulted in reoperation in 0% to 50% (median, 14%). Overall documented patient satisfaction rates varied from 39% to 100% (median, 86%). Postoperative bowel habit was only numerically quantified in 20 series, with median or mean bowel habit figures available in only 14 series (range of medians/means, 1.3–5 times per day; median, 2.9). Other functional outcome measures included diarrhea (range, 0%–46%; median, 14%); incontinence (range, 0%–52%; median, 14%); and recurrent constipation (range, 0%–33%; median, 9%). The percentage of patients still experiencing abdominal pain was documented in 14 series (range, 0%–90%; median, 41%). As a result of poor functional outcome, in particular diarrhea and incontinence or recurrent constipation, permanent ileostomy was formed in up to 28% of patients (median, 5%; range, 0%–28%). Success rates were higher in United States series (n = 11: 75%–100%, median, 94% versus Europe [65%]). Although there was no overall direct effect of length of follow-up, for study groups with results at two or more time points, successful outcomes seemed to fall off with time. Prospective studies had superior outcomes (n = 16; median, 90%; range, 50%–100%) versus retrospective studies (n = 13; median, 67%; range, 39%–100%) that may in part have been caused by more rigorous patient selection. In studies that performed anorectal physiology and transit studies, the median satisfaction rate was 89% (range, 63%–100%) versus incomplete physiology, where the median satisfaction rate was 80% (range, 39%–100%). Studies in which all patients had proved slow transit had superior outcomes to those without (median outcome, 90% versus 67%, respectively).
Since this review, several studies have continued to demonstrate similar complication rates and long-term results that vary from the very good (>80% success) to the more modest (50% success), the latter despite rigorous selection criteria. Quality of life has now also been assessed by one study using validated tools and has been shown to increase in accord with functional results.
Current position controversies
Role of segmental resection
Where selection for extent of colectomy has not been based on segmental transit studies, results for limited subtotal resections (either subtotal colectomy with cecorectal anastomosis or ileosigmoid anastomosis) have proved generally inferior to subtotal colectomy with ileorectal anastomosis. The results of segmental resection (hemicolectomy) have proved even more disappointing. Although subtotal colectomy with ileorectal anastomosis offers the best success rates, there is a perception, born out to some extent in the literature, that complications, including leakage, are more common following this anastomosis. The indication for surgery in most patients was polyposis coli or Crohn’s disease, however, and whether these results can be extrapolated to patients with constipation is unclear. In addition, functional complications, including diarrhea and urgency of defecation, may complicate this procedure and are frequently difficult to manage in clinical practice. Suboptimal function is far less problematic in patients undergoing this procedure for constipation, however, than for Crohn’s disease.
Several groups have attempted to tailor segmental surgery to pattern of transit with the use of more complex serial radiopaque marker or scintigraphic transit studies and avoid ileorectal anastomosis. De Graaf and colleagues measured segmental transit using radiopaque marker studies to select patients for partial left-sided colectomy or subtotal colectomy. Although results as a whole were disappointing, the study concluded that in terms of complications and functional outcome, there was little difference between procedures, and that a more limited resection was a reasonable option in this selected group. More recently, two studies of 40 and 28 patients have reported the use of left, right, or subtotal colectomy based on segmental transit time measurements (the latter using scintigraphy) with excellent results (82/93%). In cases where constipation recurred following segmental resection, a subtotal colectomy was undertaken successfully at a later date. Finally, the results of colonic manometry by prior colonic diversion have been used as a successful guide to surgery in 10 of 12 children. A variation on this theme has been the use of colonic manometry to select patients for subtotal colectomy, based on findings said to be consistent with colonic neuropathy.
Effect of coexistent outlet obstruction
Some studies have demonstrated a deleterious effect of untreated disorders of rectal evacuation, whereas others have not. Some groups have treated coexistent abnormalities of the pelvic floor preoperatively. A recent study of 106 patients demonstrated that despite preoperative biofeedback training, patients with nonrelaxing pelvic floor (n = 16) had significantly higher rates of recurrent defecatory difficulty, and lower rates of satisfaction after colectomy. Postoperative biofeedback has been used by others. Rectal hyposensation seems to confer a significant detrimental effect on outcome.
The effect of coexistent upper gastrointestinal dysmotility
It is generally accepted that patients with small bowel dysmotility have poor outcomes after colectomy. A fall in long-term success rate (as a result of recurrent constipation or intractable diarrhea) was demonstrated by a long-term prospective study by Redmond and coworkers (successful outcome 90%: no gastrointestinal dysmotility versus 13% gastrointestinal dysmotility) and one from Stockholm (no gastrointestinal dysmotility: 100% versus 55% with gastrointestinal dysmotility including two deaths). A high postoperative morbidity from recurrent small bowel obstruction (70%) has also been shown in such patients.
The role of laparoscopic surgery
With the safe use of laparoscopy to other areas of colorectal surgery and the disproportionately high adhesional obstruction rates after colectomy for constipation, laparoscopic colectomy has been reported in several very small case series. Only one retrospective study compared laparoscopic with open colectomy for STC with 17 patients undergoing an open and 7 a laparoscopic procedure. The laparoscopic colectomy group were more satisfied with the cosmetic outcome but had longer operation times (mean increase of 74 minutes) and increased complications. In the future, the combination of laparoscopy with tailored segmental rather than subtotal resection would have the advantage of not requiring the acknowledged technical challenges of mobilizing both colonic flexures laparoscopically.
Special considerations: idiopathic megabowel
A proportion of patients with dilatation of colon or rectum are forced to seek a surgical solution to their symptoms when conservative therapy is ineffective, is poorly tolerated, or because of complications. Numerous surgical procedures have been attempted in patients with idiopathic megabowel, including subtotal or segmental colonic resection, rectal and pelvic floor procedures, and fecal diversion. The results of these have recently undergone systematic review. In brief, colonic procedures either address the dilated bowel or have as their rationale the presentation of liquid stool to a dysfunctional rectum, which itself is not addressed (subtotal colectomy). The lower morbidity and mortality of these procedures make them attractive but functional results can be poor. Rectal procedures have higher success rates but are associated with significant morbidity and mortality (principally from intraoperative hemorrhage and pelvic sepsis). Fecal diversion remains a possibility but is an unattractive prospect in young patients. Two procedures that are gaining greater recognition are restorative proctocolectomy with ileal pouch formation (outcome reported in 22 patients in the literature, being successful in 73% [range, 57%–100%] ) and a novel procedure, the vertical reduction rectoplasty. The latter, at medium-term (5-year) follow-up, was successful in achieving and maintaining correction of rectal diameter, compliance, and sensory function in most of 10 patients, and this was translated into clinical benefit with no operative mortality and minimal morbidity.
Anterograde colonic enema
The anterograde colonic enema technique was first described by Malone and coworkers in 1990 using the appendix as a conduit in children with neuropathic constipation. Various modifications have subsequently been described.
Rationale
The purpose is to maintain efficient emptying of the lower bowel through regular irrigation of water or saline, with or without aperients, by a catheter inserted into the proximal colon.
Indications and patient selection
The use of anterograde colonic enema should be considered as an alternative to colectomy or stoma when conservative methods of laxatives have failed and more radical surgery is to be avoided because of recognized lack of efficacy (particularly severe outlet obstruction) or unacceptability (eg, in children). In patients with previous appendicectomy or in whom the appendix cannot be satisfactorily used, cecostomy may be effected using a percutaneously placed Chait tube or surgically by more complex techniques, such as stapled tubularized cecal neoappendicostomy or continent colonic conduit.
Results and complications
In general, success rates have been lower in adults than in children. Despite early functional success, in the long term, such complications as stomal stenosis and leakage, or failure effectively to treat symptoms, commonly (>50% at 3 years) lead to revision, reversal, or conversion to stoma.
Current position controversies
The role of anterograde colonic enema in adults is less clear than in children. There are no trials comparing anterograde colonic enema with other therapies for constipation in any group of patients.
Stoma
A stoma may be used as a definitive procedure, as a guide to further treatment, or as salvage from failed or complicated prior surgical intervention. There are little published data to support an evidence-based use; however, the suggestion that an ileostomy can guide the use of colectomy is an approach used by the authors and others when subsequent colectomy would be avoided if the ileostomy output is unsatisfactorily high or symptoms, such as pain and bloating untouched by diversion. As a definitive procedure both colostomy and ileostomy have been described for a diversity of adult and childhood disorders characterized by constipation including spinal cord injury, megacolon, and outlet obstruction. There is little evidence to guide choice of ileostomy or colostomy ; however, some report high complication rates of ileostomy and STC may be unsatisfactorily treated by colostomy.
Evolving Surgical Treatments
Sacral nerve stimulation
First applied in urology and thence FI (see later), SNS is increasingly being considered as the first-line procedural intervention in constipation after failure of conservative measures.
Rationale
As in FI, the mechanism of action of SNS is not conclusively proved and may involve direct effects on colorectal sensory or motor function or central effects at the level of spinal cord or brain.
Indications
Although yet to be clearly established, SNS will probably come to have a role in idiopathic constipation (with or without proved transit disturbance) that is resistant to conservative treatment (laxatives, behavioral modification) before more radical measures, such as resectional surgery or anterograde enema, are considered.
Results
Although sacral root (parasympathetic) Brindley stimulation was described for neurogenic constipation in 1991, it was not until 2002 that the St. Mark’s group first described the application of SNS in its current form in three pilots of small numbers of patients with severe idiopathic constipation, some of whom had STC. From a clinical perspective, these demonstrated (1) symptomatic benefit of temporary stimulation for a 3-week period in two of eight patients with STC; (2) symptomatic and quality–of-life benefits of permanent stimulation in three of four patients (two with STC); and (3) demonstration of efficacy with a crossover design of two patients with stimulation “on” and “off”. A recent multicenter European study of 65 patients with normal or STC has subsequently built on these results with 43 (66%) patients going on to permanent stimulation on the basis of 50% symptom improvement. In this group, there were significant improvements in nearly all symptoms and quality-of-life measures. The relatively high success rate in this study should be tempered by a very recent study of 19 patients with mixed STC and outlet obstruction that demonstrated a more modest success rate of 42%.
From a mechanistic perspective, the earlier temporary stimulation study of Malouf and colleagues, which comprised eight patients with STC, showed no effect on colonic transit times even in the two patients with improved symptoms. This contrasted with another mechanistic study, in which six of eight patients with STC benefited from SNS. In this study, stimulation caused significant alterations in the incidence of colonic high-amplitude propagating contractions, the main functional correlate of which is thought to be mass movements of stool, and whose incidence has been shown to be reduced in patients with STC compared with control subjects. In the larger European study, transit times were normalized in one half of patients with STC at baseline. More consistent is the effect of SNS to improve rectal sensory function. This has been well reported for FI and is also of interest mechanistically given that a significant proportion of patients with STC or outlet obstruction have reduced perception of rectal filling often with accompanying loss of defecatory urge (ie, rectal hyposensitivity).
Current position controversies
Common to both studies of FI and constipation is the rather arbitrary definition of success as a 50% improvement in symptoms and the subsequent failure of study design to incorporate intention-to-treat analysis. Long-term data are still lacking in respect to constipation. Nevertheless, as suggested by a recent Cochrane review, the limited evidence suggests that the procedure holds promise for selected patients. The effects on reducing bloating and abdominal pain (poorly addressed by other modalities of treatment) in most studies suggest that patients in which these symptoms predominate may perhaps be benefited most (although this requires confirmation).
Stapled transanal rectal resection: STARR
Following the introduction of stapled hemorrhoidopexy in the 1980s, there has been recent interest in using circular staplers in the management of obstructed defecation. This procedure, although perhaps also addressing anatomic abnormalities excluded from this article (eg, rectocele, prolapse, intussusception), is briefly described on the basis that obstructed defecation (with constipation) may be treated in some patients who have no such evident proctologic abnormalities.
Rationale
Obstructed defecation encompasses a number of symptoms that in part or whole may relate to a number of clinical and physiologic findings, such as perineal descent, rectocele, and intussusception. The stapled transanal rectal resection procedure resects internally prolapsed rectum with the aim of improved function (perhaps through improved rectal compliance and sensation and volume).
Indications
The procedure is indicated for failed prior conservative management in patients with characteristic symptoms and clinical or physiologic findings of obstructed defecation. A number of specific exclusion criteria also exist.
Results
In the last 4 years, several publications have attested to the successful results of this procedure in treating obstructed defecation symptoms (eg, 88% in one study). The procedure has the advantage of minimal postoperative pain; however, numerous quite serious complications (eg, fistula) have been described in up to 50% of patients.
Current position controversies
There is a requirement to define criteria better for the procedure and long-term results. Complications remain a concern.
Surgical treatment of fecal incontinence
Introduction
Most patients with mild to moderate symptoms successfully respond to conservative management, and this must be considered as first-line therapy. Therapeutic strategies comprise pharmacologic, behavioral, and physical modalities. Only patients who fail to respond to such measures (ie, those with severe symptoms or major incontinence) should be referred to a specialist tertiary center for further investigation and consideration for surgical intervention. The surgical management of FI is often complex, and in common with any surgical procedure has its own inherent risks and complications. It should be reserved for patients with severe incontinence with impaired quality of life who have failed, or are deemed unsuitable for nonsurgical management. The management of patients with FI in a specialist surgical unit involves a multidisciplinary team of professionals. Because patient selection is crucial for a successful outcome, the importance of a thorough and comprehensive clinical and physiologic assessment cannot be overstressed. Because no form of surgical intervention offers certainty of cure, preoperatively counseling before surgery is obligatory.
The following description concentrates specifically on the choice of surgical procedure appropriate to the underlying pathophysiology of the incontinence. Detailed, objective assessment yields four broad clinical categories of patients with FI. These include patients with predominantly (1) simple, structural defects of the anal sphincters; (2) weak but intact anal sphincters; (3) complex disruption of the anal sphincter complex; and (4) extrasphincteric abnormalities. The surgical interventions available to address such abnormalities and their outcomes are shown in Table 2 .
Historical: Correction of Abnormalities of the Pelvic Floor
Traditionally, pelvic floor procedures (postanal repair, anterior levatorplasty, total pelvic floor repair) have been performed for patients without a specific sphincter defect who suffer with idiopathic or neurogenic incontinence. Such procedures involved plication of various components of the pelvic floor musculature (levator ani, puborectalis, and the external anal sphincter [EAS]) to reconstitute the anorectal angle and lengthen the anal canal. Follow-up studies following postanal repair and anterior levatorplasty have revealed disappointing results with typically only one third to one half of patients having improved continence, with no observed difference between the two procedures in comparative studies. Total pelvic floor repair involves a combination of postanal repair with anterior levatorplasty and sphincter plication, but only achieves improved continence and quality of life in approximately one half of all patients. A recent Cochrane review of randomized trials has confirmed no difference in numbers of patients achieving full continence in anterior levatorplasty compared with postanal repair, total pelvic floor repair compared with anterior levatorplasty, and total pelvic floor repair compared with postanal repair. Although once popular in the United Kingdom and Europe, pelvic floor procedures are now far less frequently performed.
Contemporary
Correction of abnormalities and augmentation of the native anal sphincter complex
Sphincteroplasty
Surgery to the anal sphincter complex has largely been confined to repair of EAS defects, because repair of isolated internal sphincter defects has not proved successful in patients with passive fecal soiling. Anterior EAS defects usually follow obstetric trauma, and may be repaired immediately if identified at the time of injury. Delayed repair is more frequently performed, however, because of unrecognized injury or failure of primary repair. Such repair may involve direct apposition, or overlapping, of the edges of the disrupted sphincter.
Rationale
Sphincteroplasty aims to restore anatomic integrity and function to a disrupted EAS.
Indications and patient selection
Direct repair is appropriate in patients with isolated defects affecting one third or less of the circumference of the EAS on endosonography.
Results and complications
Most studies addressing the functional outcome of sphincteroplasty report early success rates of 70% to 90%, but are generally restricted to short-term follow-up. Reported success rates at 5 years fall to approximately 50%. Furthermore, some patients may develop problematic evacuation disorders.
Current position controversies
- 1.
Redo sphincteroplasty following failed repair. Given the 70% to 90% short-term and 50% long-term success rates of sphincteroplasty, it is clear that some patients have persistent FI after surgery. Because many of these patients have residual anterior sphincter defects, it is possible to perform a repeat sphincter repair. The outcome of repeat sphincter repair does not seem to be affected by previous surgery, and is associated with significant improvements in patient continence scores.
- 2.
Overlapping versus direct sphincter repair. Previously, sphincter repair was performed by apposition of the separated edges of the external sphincter. More recently, superior results have been suggested if an overlapping repair is performed, which is now largely considered the operation of choice for definable sphincter defects. A recent Cochrane review has revealed that outcome is the same, however, regardless of whether the sphincter repair is direct or overlapping.
- 3.
Influence of clinical and physiologic factors on outcome of repair. It has been suggested that certain factors, such as patient age and pudendal nerve function, may be important in predicting outcome following surgery, although the literature is largely contradictory in this regard. Some studies have reported increasing age (especially in those >50 years) as a predictor of failure. In contrast, others have found no influence of age on functional outcome or even a superior functional outcome in patients older than 50 years compared with their younger counterparts. Controversy also exists relating to pudendal nerve function. Several studies have implicated pudendal neuropathy as a predictor of failure following sphincteroplasty. Other studies have failed to identify any relationship and conclude that repair of anatomic sphincter defects should still be considered in the presence of pudendal neuropathy.
Sacral nerve stimulation
Rationale
The exact mechanism of action of SNS remains unclear with the initial, and intuitive, premise that SNS would directly augment anal sphincter function and improve FI now questioned by more detailed physiologic studies. Indeed, the observation that improved continence occurred without change in anal sphincter function has led to the suggestion that SNS has predominantly suprasphincteric effects. The mechanism of action of SNS is not conclusively proved and may involve direct effects peripherally on colorectal sensory or motor function, or central effects at the level of spinal cord or brain.
Indications and patient selection
Early studies of SNS in FI restricted inclusion of patients to those with a functionally deficient but morphologically intact anal sphincter. With increasing experience of the technique the inclusion criteria have extended to include patients with EAS defects; internal anal sphincter (IAS) defects; and FI secondary to cauda equina syndrome and (partial) spinal injuries.
Results and complications
Initial reports of SNS detailed successful short-term results in small numbers of patients, with marked improvements in clinical symptoms associated with improvement of physiologic parameters, such as anal canal pressures and rectal sensory function. Significant improvements in quality of life have been demonstrated using both generic and incontinence-specific measures. The results of multicenter studies of SNS also support these findings, with a marked, unequivocal improvement in FI and patient quality of life, with such improvements being sustained in the medium term (24 months).
A recent systematic review of the published outcomes of trials investigating SNS revealed that 40% to 75% of patients achieved complete fecal continence and 75% to 100% experienced improvement in episodes of incontinence, with a low (10%) incidence of adverse events. Further confirmation of the effectiveness of SNS in the treatment of FI was recently demonstrated in a randomized, controlled trial where it was found to be superior to treatment with best supportive therapy (pelvic floor exercises, bulking agents, and dietary manipulation) in terms of improvement in fecal continence and the FI quality-of-life scores. The availability of this level 1 evidence, which is lacking for most surgical procedures for FI, allows SNS to be considered as a contemporary treatment option for FI rather than one that is evolving.
Current position controversies
- 1.
Reporting of outcomes and definition of success. As noted in the constipation section, the arbitrary definition of success as being a 50% reduction in incontinence episodes, while satisfying the indications for permanent stimulation, is of little comfort to the patient who may still have significant, albeit reduced, episodes of FI. Again, the failure to account for drop-outs (ie, those patients not going on to permanent stimulation) in the reporting of outcomes (ignoring the convention of clinical trials to use intention-to-treat analyses) should be noted.
- 2.
Long-term results. Current outcome studies of SNS are limited to medium-term follow-up, and whether such benefit is maintained in the longer-term is currently unknown. Accordingly, patients should be counseled appropriately until long-term data are available.
- 3.
Range of indications for SNS in FI. There are some preliminary data suggesting that SNS can be successfully applied to patients with FI secondary to neurologic conditions and those with anal sphincter defects. Indeed, there is a growing body of evidence that suggests that patients with de novo EAS defects or defects after unsuccessful previous sphincter repair receive benefit from SNS and that there is no difference in medium-term outcome between patients with EAS defects and patients with intact anal sphincter muscles. The contemporary view is that a morphologically intact anal sphincter is not a prerequisite for success in the treatment of FI with SNS and that EAS defects of less than 33% of the circumference can be effectively treated primarily without repair.
Creation of a new anal sphincter (neosphincter)
Electrically stimulated (dynamic) graciloplasty
Encirclement procedures involving transposition of skeletal muscle around the anus to create a neosphincter have been performed for many years. The gluteus maximus, adductor longus, and obturator internus muscles have all been used, although the gracilis muscle is the favored option, being the most superficial medial adductor muscle with a sufficiently plastic neurovascular supply.
Rationale
Gracilioplasty involves reconstruction of the anal sphincter using native skeletal muscle. When first performed, improvements in continence were dependent on causing a degree of anal canal obstruction and the results were generally poor. The procedure gained popularity following description of the dynamic graciloplasty, however, which involved the application of chronic low-frequency electrical stimulation to the muscle by a subcutaneously placed generator, transforming it to a slow-twitch nonfatigable muscle capable of a tonic state of contraction. The electrode lead is connected to the stimulator, which is implanted subcutaneously. On-off function of the stimulator, allowing defecation to take place, is governed by a hand-held magnet.
Indications and patient selection
The procedure is reserved for carefully selected patients in whom the anal sphincter musculature is irreparable and who are desperate to avoid a stoma. This includes those with disrupted anal sphincters that are unsuitable for or have already failed the procedures discussed previously, together with those who have a damaged or absent (often as a result of congenital defects) IAS resulting in severe passive leakage, refractory to all other treatments.
Results and complications
In prospective multicenter trials, between 56% and 72% of patients undergoing dynamic graciloplasty have achieved and maintained a successful outcome, with the best outcomes observed in those with traumatic incontinence, with similar success rates (42%–85%) observed in a recent systematic review. The only outcomes have thus far been reported by centers with a particular interest in the procedure, where continence to at least solid and liquid stool has been reported in approximately 70% of all patients at median-term follow-up. It should be noted, however, that this procedure has never been the subject of controlled or comparative trials.
Dynamic graciloplasty operations are technically demanding and may be associated with high morbidity, with infection reported in up to one third of cases. In addition, postoperative evacuatory disorders occur in up to one quarter of patients and are more difficult to resolve. It seems likely that the procedure should be confined to specialist colorectal centers, and reserved for carefully selected patients.
Artificial bowel sphincter
Anal sphincter reconstruction can also be performed using a synthetic sphincter device. Having been used for many years in the treatment of urinary incontinence, the first successful use of an artificial sphincter for the treatment of FI was in 1987.
Rationale
The contemporary device consists of an inflatable silicon cuff, which is implanted around the anal canal and controlled by the patient by a pump located in the scrotum or labium majus. Activation of the pump forces fluid from the cuff into a reservoir implanted suprapubically in the space of Retzius, deflating the cuff and allowing defecation. Subsequently, the cuff automatically reinflates slowly to maintain continence until the next evacuation.
Indications and patient selection
The indications for use of this procedure are broadly the same as for dynamic graciloplasty. It may additionally be used in FI of neuromuscular origin (eg, myasthenia gravis and neuropathy secondary to diabetes mellitus).
Results and complications
Several groups have reported their experiences with the artificial bowel sphincter in small numbers of patients with overall improvements in continence in approximately one half to three quarters of patients. Further, artificial bowel sphincter, unlike dynamic graciloplasty, has been the subject of a prospective, randomized, controlled trial where it was demonstrated to be better than conservative treatment in improving continence. Long-term outcome seems less encouraging, however, with two studies with a median follow-up of approximately 7 years documenting success rates of less than 50%, explantation rates as high as 49%, and infection in up to one third of cases. As with dynamic gracilioplasty, there seems to be a high incidence of postoperative evacuatory difficulties, which are present in up to one half of patients.
End-stage and refractory fecal incontinence
Permanent end stoma
As for constipation, fecal diversion usually by colostomy is an option in end-stage, devastating FI affecting quality of life. Most patients (83%) who have a permanent end colostomy fashioned for FI report that the stoma restricted their life “a little” or “not at all” and would “probably” or “definitely” choose to have the stoma again, although overall quality of life, assessed using generic measures, was poor. A few had not adapted, however, and disliked the stoma intensely. Furthermore, there may be a high rate of stoma complications.
Evolving
Correction of abnormalities and augmentation of the native anal sphincter complex
Perianal injection of biomaterials
Perianal injection of various bulking agents has been performed in patients with FI. Numerous biomaterials have been injected, details of which can be found in a recent review article; most experience and success has been achieved with silicone.
Rationale
The injection of biomaterials physically augments the (internal) anal sphincter.
Indications and patient selection
It is indicated in FI caused by a weak or disrupted IAS or patients with passive fecal soiling.
Results and complications
Injection of silicone seems to be associated with an improvement in fecal continence and quality of life in patients with internal sphincter dysfunction, with approximately two thirds of patients showing either marked improvement or complete cessation of leakage in the short term. Longer term (5 years) revealed little change in their incontinence score compared with before the procedure, however, with one of six patients requiring a colostomy for FI and another for a rectovaginal fistula. The considerable morbidity of this procedure has cast serious doubts on the use of this intervention in patients with FI, although it may be considered in carefully counseled patients with passive FI secondary to IAS dysfunction or defects in whom treatment options are otherwise limited.
Radiofrequency energy delivery to the anal canal (SECCA procedure)
Temperature-controlled radiofrequency energy has been delivered deep to the mucosa of the anal canal by multiple needle electrodes using a specially designed anoscopic handpiece inserted into the anal canal in patients with FI.
Rationale
The proposed mechanism of action is by heat-induced tissue contraction and remodeling of the anal canal and distal rectum.
Indications and patient selection
Recruitment of patients into studies to date has included those with FI refractory to standard medical therapy from varying causes.
Results and complications
In the first reported case series, 8 of 10 patients responded to the treatment and the modality was found to be safe and associated with improved continence and quality of life scores. Symptomatic improvement was sustained at 2 years and recently published medium-term data reveal significant and sustained improvements in symptoms of FI and quality of life are seen at 5 years after treatment. A multicenter trial also confirms the improvements in FI and quality of life at least in the short term (at 6-month follow-up). Complications included ulceration of the mucosa and delayed bleeding. There were no changes in the results of anal manometry pudendal nerve terminal motor latencies or endoanal ultrasound. A randomized trial to determine its role in the treatment of FI has been recently completed in the United States, and the data should be available soon. Further studies of greater numbers of patients are required before its widespread use can be recommended.
Correction of extrasphincteric physiologic abnormalities: dysfunction rectal reservoir
In addition to occurring secondary to anal sphincter dysfunction, there is increasing awareness that FI may also result from suprasphincteric causes, such as dysfunction of the rectal reservoir. This dysfunction may manifest as an impaired ability to store or evacuate feces. Reservoir dysfunction may occur in isolation, or combination with other pathophysiologic abnormalities, and may complicate surgical procedures designed to improve continence. The anterograde colonic enema may also be used to overcome reservoir dysfunction in patients with (overflow) FI secondary to impaired rectal evacuation.
Rectal augmentation for storage dysfunction
Rectal augmentation is a novel approach to correct physiologic abnormalities in a subgroup of patients with intractable FI secondary to reservoir dysfunction (akin to the clam enterocystoplasty for the treatment of the overactive bladder). Even in the absence of anal sphincter dysfunction, patients may still suffer with severe urgency of defecation and urge incontinence secondary to derangements of rectal sensorimotor function. Such patients have low rectal compliance and heightened rectal sensation (rectal hypersensitivity). Management is problematic, because correction of the sphincter defect does not abolish the incapacitating urgency or incontinence.
Rationale
A novel procedure was developed to treat selected patients presenting with incapacitating urgency and FI by specifically addressing the underlying pathophysiologic abnormalities. The procedure involves the creation of a side-to-side ileorectal pouch, or ileorectoplasty, which involves incorporating a 10-cm patch of ileum on its vascular pedicle into the anterior rectal wall ( Fig. 2 ) to increase its capacity and compliance and restore rectal sensitivity to normal.









